TesaglitazarCAS# 251565-85-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 251565-85-2 | SDF | Download SDF |
| PubChem ID | 208901 | Appearance | Powder |
| Formula | C20H24O7S | M.Wt | 408.47 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | AZ 242 | ||
| Solubility | Soluble to 100 mM in DMSO and to 100 mM in ethanol | ||
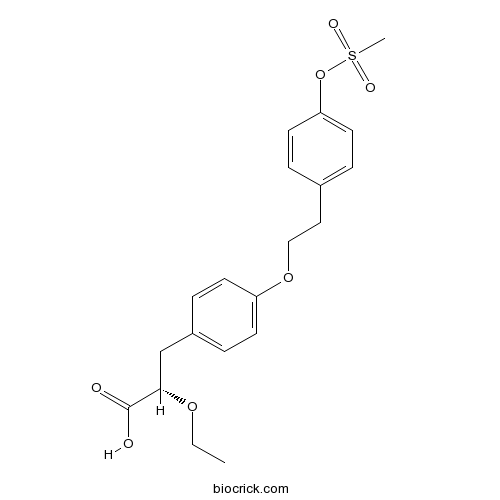
| Chemical Name | (2S)-2-ethoxy-3-[4-[2-(4-methylsulfonyloxyphenyl)ethoxy]phenyl]propanoic acid | ||
| SMILES | CCOC(CC1=CC=C(C=C1)OCCC2=CC=C(C=C2)OS(=O)(=O)C)C(=O)O | ||
| Standard InChIKey | CXGTZJYQWSUFET-IBGZPJMESA-N | ||
| Standard InChI | InChI=1S/C20H24O7S/c1-3-25-19(20(21)22)14-16-6-8-17(9-7-16)26-13-12-15-4-10-18(11-5-15)27-28(2,23)24/h4-11,19H,3,12-14H2,1-2H3,(H,21,22)/t19-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Dual-specificity PPARα/γ agonist (IC50 values are 0.35 and 3.8 μM for PPARγ and PPARα respectively). Prevents atherosclerosis progression in E3L.CETP transgenic mice. Also reduces insulin resistance in obese Zucker rats. Orally active. |

Tesaglitazar Dilution Calculator

Tesaglitazar Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4482 mL | 12.2408 mL | 24.4816 mL | 48.9632 mL | 61.204 mL |
| 5 mM | 0.4896 mL | 2.4482 mL | 4.8963 mL | 9.7926 mL | 12.2408 mL |
| 10 mM | 0.2448 mL | 1.2241 mL | 2.4482 mL | 4.8963 mL | 6.1204 mL |
| 50 mM | 0.049 mL | 0.2448 mL | 0.4896 mL | 0.9793 mL | 1.2241 mL |
| 100 mM | 0.0245 mL | 0.1224 mL | 0.2448 mL | 0.4896 mL | 0.612 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- M344
Catalog No.:BCC2162
CAS No.:251456-60-7
- SU 16f
Catalog No.:BCC7639
CAS No.:251356-45-3
- Urotensin II (human)
Catalog No.:BCC5796
CAS No.:251293-28-4
- Crebanine
Catalog No.:BCN5117
CAS No.:25127-29-1
- FRATide
Catalog No.:BCC5821
CAS No.:251087-38-4
- Antazoline HCl
Catalog No.:BCC4627
CAS No.:2508-72-7
- Salvisyrianone
Catalog No.:BCN4821
CAS No.:250691-57-7
- NNC 63-0532
Catalog No.:BCC7177
CAS No.:250685-44-0
- Pedunsaponin A
Catalog No.:BCN8192
CAS No.:250613-27-5
- Cyclo(RGDyK)
Catalog No.:BCC6512
CAS No.:250612-42-1
- (±)-Acetylcarnitine chloride
Catalog No.:BCC6617
CAS No.:2504-11-2
- Excavatin M
Catalog No.:BCN5116
CAS No.:250293-31-3
- 5-Chloro-2-nitrobenzoic acid
Catalog No.:BCC8743
CAS No.:2516-95-2
- Acevaltrate
Catalog No.:BCN7127
CAS No.:25161-41-5
- Loline
Catalog No.:BCN2003
CAS No.:25161-91-5
- Isohomoarbutin
Catalog No.:BCN7612
CAS No.:25162-30-5
- Dynamin inhibitory peptide
Catalog No.:BCC1034
CAS No.:251634-21-6
- AM 1172
Catalog No.:BCC7675
CAS No.:251908-92-6
- SLV 320
Catalog No.:BCC7656
CAS No.:251945-92-3
- A 205804
Catalog No.:BCC3944
CAS No.:251992-66-2
- AZD7545
Catalog No.:BCC4294
CAS No.:252017-04-2
- Fmoc-D-Threoninol
Catalog No.:BCC2702
CAS No.:252049-02-8
- Fmoc-Lys(Me2)-OH
Catalog No.:BCC2567
CAS No.:252049-10-8
- Tertiapin-Q
Catalog No.:BCC5740
CAS No.:252198-49-5
PPAR Agonists: II. Fenofibrate and Tesaglitazar Alter Behaviors Related to Voluntary Alcohol Consumption.[Pubmed:26857541]
Alcohol Clin Exp Res. 2016 Mar;40(3):563-71.
BACKGROUND: In the accompanying article, we showed that activation of peroxisome proliferator-activated receptor alpha (PPARalpha) signaling by fenofibrate and Tesaglitazar decreases ethanol (EtOH) consumption in mice. In this study, we determined the role of these PPAR agonists in EtOH-related behaviors and other actions that may be important in regulating EtOH consumption. METHODS: The effects of fenofibrate (150 mg/kg) and Tesaglitazar (1.5 mg/kg) were examined on the following responses in male and female C57BL/6J (B6) and B6 x 129S4 mice: preference for saccharin, EtOH-induced conditioned place preference (CPP), conditioned taste aversion (CTA), loss of righting reflex, and withdrawal, acoustic startle reflex, response to novelty, and EtOH clearance. Because the B6 inbred strain usually displays weak EtOH-induced CPP and weak EtOH-induced acute withdrawal, B6 x 129S4 mice were also studied. RESULTS: Fenofibrate and Tesaglitazar decreased the novelty response and increased acute EtOH withdrawal severity, and fenofibrate increased EtOH-induced CTA. Two important factors for EtOH consumption (saccharin preference and EtOH-induced CPP) were not altered by fenofibrate or Tesaglitazar. EtOH clearance was increased by both fenofibrate and Tesaglitazar. Response to novelty, acute withdrawal, and EtOH clearance show sex differences and could contribute to the reduced EtOH consumption following fenofibrate administration. CONCLUSIONS: These studies indicate the complexity of EtOH-dependent and EtOH-independent behaviors that are altered by PPAR agonists and provide evidence for novel behavioral actions of these drugs that may contribute to PPAR-mediated effects on alcohol drinking.
[Peroxisome proliferator-activated receptor alpha/gamma agonist tesaglitazar stabilizes atherosclerotic plaque in diabetic low density lipoprotein receptor knockout mice].[Pubmed:23710746]
Zhonghua Xin Xue Guan Bing Za Zhi. 2013 Feb;41(2):143-9.
OBJECTIVE: To investigate the effects of peroxisome proliferator-activated receptor (PPAR) alpha/gamma agonist on atherosclerotic plaque stabilization in diabetic LDL receptor knockout (LDLr-/-) mice. METHODS: Female 4-week-old LDLr-/- mice fed with high-glucose and high-fat diet for 4 weeks were randomly divided into three groups (n = 15 each): control group (only fed with high-glucose and high-fat diet), diabetic group [induced by high-glucose and high-fat diet combined with a low-dose of streptozotocin (STZ)] without Tesaglitazar and with Tesaglitazar (20 microg/kg oral treatment). After 6 weeks, the mice were sacrificed, body weight, fasting blood glucose (Glu), total cholesterol (TC), triglyceride (TG) levels were measured. The expression of ICAM-1, VCAM-1, MCP-1 in the brachiocephalic atherosclerotic lesions were determined by Western blot and immunohistochemistry, respectively. Brachiocephalic artery was prepared for morphologic study (HE, oil red O, Sirius red staining) and immunohistochemical analysis (macrophage surface molecule-3, alpha-smooth muscle actin), respectively. RESULTS: Serum TC [(32.34 +/- 3.26) mmol/L vs. (16.17 +/- 1.91) mmol/L], TG [(3.57 +/- 0.99) mmol/L vs. (2.21 +/- 0.11) mmol/L] and Glu [(15.21 +/- 4.67) mmol/L vs. (6.89 +/- 0.83) mmol/L] levels were significantly higher in diabetic group than in the control group (all P < 0.01). The expression of ICAM-1 (2.31 +/- 0.35 vs.1.34 +/- 0.21), VCAM-1 (1.65 +/- 0.14 vs.0.82 +/- 0.26), MCP-1 (2.27 +/- 0.16 vs.1.56 +/- 0.23) were significantly upregulated in diabetic group compared with control group (all P < 0.01). Brachiocephalic atherosclerotic plaque area [(4.597 +/- 1.260)x10(3) microm(2) vs. (0.075 +/- 0.030)x10(3) microm(2)], lipid deposition [(47.23 +/- 2.64)% vs. (9.67 +/- 1.75)%], Mac-3 positive area [(19.15 +/- 3.51)% vs. (1.72 +/- 0.16)%], alpha-smooth muscle actin [(5.54 +/- 1.17)% vs. (2.13 +/- 0.41)%] and collagen content [(4.27 +/- 0.74)% vs. (0.43 +/- 0.09)%] were all significantly larger/higher in diabetic LDLr-/- mice than in the control group (all P < 0.01). While Tesaglitazar treatment significantly reduced serum TC [(30.47 +/- 3.18) mmol/L], TG [(3.14 +/- 0.71) mmol/L] and Glu [(7.92 +/- 1.28) mmol/L] levels (all P < 0.01). Similarly, the expression of ICAM-1 [(1.84 +/- 0.22)], VCAM-1 [(1.27 +/- 0.11)], MCP-1 [(1.83 +/- 0.24)], brachiocephalic atherosclerotic lesion area[(1.283 +/- 0.410)x10(3) microm(2)], lipid deposition[(23.52 +/- 1.39)%] were also significantly reduced by Tesaglitazar (all P < 0.05). Moreover, Tesaglitazar increased alpha-smooth muscle actin [(9.46 +/- 1.47)%] and collagen content [(6.32 +/- 1.15)%] in diabetic LDLr-/- mice (all P < 0.05). In addition, lipid deposition and Mac-3 positive areas [(10.67 +/- 0.88)% vs. (15.83 +/- 1.01)%] in the aortic root were also reduced in Tesaglitazar treated diabetic LDLr-/- mice (P < 0.01). CONCLUSIONS: Tesaglitazar has anti-inflammatory effects in the diabetic LDLr-/- mice. Tesaglitazar could reduce lipid deposition, increase collagen and alpha-SMA content in the brachiocephalic atherosclerotic lesions, thus, stabilize atherosclerotic plaque in this model.
Tesaglitazar ameliorates non-alcoholic fatty liver disease and atherosclerosis development in diabetic low-density lipoprotein receptor-deficient mice.[Pubmed:23226761]
Exp Ther Med. 2012 Dec;4(6):987-992.
Previous research has demonstrated that the dual PPARalpha/gamma agonist Tesaglitazar reduces atherosclerosis in a mouse model of hyperlipidemia by reducing both lipid content and inflammation in the aorta. However, much of the underlying mechanism of Tesaglitazar in non-alcoholic fatty liver disease (NAFLD) remains less clear. The aim of the present study was to determine whether Tesaglitazar attenuates NAFLD and atherosclerosis development in diabetic low-density lipoprotein receptor-deficient (LDLr(-/-)) mice. Female LDLr(-/-) mice (3 weeks old) were induced by a high-fat diet (HFD) combined with low-dose streptozotocin (STZ) injection to develop an animal model of type 2 diabetes (T2DM). The mice were randomly divided into two groups: diabetic group (untreated diabetic mice, n=15) and Tesaglitazar therapeutic group (n=15, 20 mug/kg/day oral treatment for 6 weeks). Fifteen LDLr(-/-) mice were fed with an HFD as the control group. Tesaglitazar decreased serum glucose and lipid levels compared with the diabetic mice. Tesaglitazar significantly reduced atherosclerotic lesions, lipid accumulation in the liver, macrophage infiltration, and decreased total hepatic cholesterol and triglyceride content compared to the diabetic mice. In addition, Tesaglitazar reduced inflammatory markers at both the serum and mRNA levels. Our data suggest that Tesaglitazar may be effective in preventing NAFLD and atherosclerosis in a pre-existing diabetic condition by regulating glucose and lipid metabolism, and the inflammatory response.
The PPAR alpha / gamma Agonist, Tesaglitazar, Improves Insulin Mediated Switching of Tissue Glucose and Free Fatty Acid Utilization In Vivo in the Obese Zucker Rat.[Pubmed:24285952]
PPAR Res. 2013;2013:305347.
METABOLIC FLEXIBILITY WAS ASSESSED IN MALE ZUCKER RATS: lean controls, obese controls, and obese rats treated with the dual peroxisome proliferator activated receptor (PPAR) alpha/gamma agonist, Tesaglitazar, 3 mu mol/kg/day for 3 weeks. Whole body glucose disposal rate (R d ) and hepatic glucose output (HGO) were assessed under basal fasting and hyperinsulinemic isoglycemic clamp conditions using [3,(3)H]glucose. Indices of tissue specific glucose utilization (R g ') were measured at basal, physiological, and supraphysiological levels of insulinemia using 2-deoxy-D-[2,6-(3)H]glucose. Finally, whole body and tissue specific FFA and glucose utilization and metabolic fate were evaluated under basal and hyperinsulinemic conditions using a combination of [U-(13)C]glucose, 2-deoxy-D-[U-(14)C]glucose, [U-(14)C]palmitate, and [9,10-(3)H]-(R)-bromopalmitate. Tesaglitazar improved whole body insulin action by greater suppression of HGO and stimulation of R d compared to obese controls. This involved increased insulin stimulation of R g ' in fat and skeletal muscle as well as increased glycogen synthesis. Tesaglitazar dramatically improved insulin mediated suppression of plasma FFA level, whole body turnover (R fa ), and muscle, liver, and fat utilization. At basal insulin levels, Tesaglitazar failed to lower HGO or R fa compared to obese controls. In conclusion, the results demonstrate that Tesaglitazar has a remarkable ability to improve insulin mediated control of glucose and FFA fluxes in obese Zucker rats.
The dual PPARalpha/gamma agonist tesaglitazar blocks progression of pre-existing atherosclerosis in APOE*3Leiden.CETP transgenic mice.[Pubmed:19220285]
Br J Pharmacol. 2009 Apr;156(7):1067-75.
BACKGROUND AND PURPOSE: We have evaluated the effects of a peroxisome proliferator-activated receptor (PPAR)alpha/gamma agonist on the progression of pre-existing atherosclerotic lesions in APOE*3Leiden.cholesteryl ester transfer protein (E3L.CETP) transgenic mice. EXPERIMENTAL APPROACH: E3L.CETP mice were fed a high-cholesterol diet for 11 weeks to induce atherosclerosis, followed by a low-cholesterol diet for 4 weeks to obtain a lower plasma total cholesterol level of approximately 10 mmol.L(-1). Mice were divided into three groups, which were either killed before (baseline) or after an 8 week treatment period with low-cholesterol diet without (control) or with the PPARalpha/gamma agonist Tesaglitazar (10 microg.kg(-1).day(-1)). Atherosclerosis was assessed in the aortic root. KEY RESULTS: Treatment with Tesaglitazar significantly reduced plasma triglycerides, total cholesterol, CETP mass and CETP activity, and increased high-density lipoprotein-cholesterol. At baseline, substantial atherosclerosis had developed. During the 8 week low-cholesterol diet, atherosclerosis progressed in the control group with respect to lesion area and severity, whereas Tesaglitazar inhibited lesion progression during this period. Tesaglitazar reduced vessel wall inflammation, as reflected by decreased monocyte adhesion and macrophage area, and modified lesions to a more stabilized phenotype, with increased smooth muscle cell content in the cap and collagen content. CONCLUSIONS AND IMPLICATIONS: Dual PPARalpha/gamma agonism with Tesaglitazar markedly improved the atherogenic triad by reducing triglycerides and very low-density lipoprotein-cholesterol and increasing high-density lipoprotein-cholesterol and additionally reduced cholesterol-induced vessel wall activation. These actions resulted in complete inhibition of progression and stabilization of pre-existing atherosclerotic lesions in E3L.CETP mice.
Synthesis and biological and structural characterization of the dual-acting peroxisome proliferator-activated receptor alpha/gamma agonist ragaglitazar.[Pubmed:12672231]
J Med Chem. 2003 Apr 10;46(8):1306-17.
A new and improved synthesis of the peroxisome proliferator-activated receptor (PPAR) agonist ragaglitazar applicable for large-scale preparation has been developed. The convergent synthetic procedure was based on a novel enzymatic kinetic resolution step. The conformation of ragaglitazar bound to the hPPARgamma receptor was quite different compared to the single-crystal structures of the l-arginine salt of ragaglitazar. In particular, the phenoxazine ring system had varying orientations. Ragaglitazar had high affinity for the hPPARalpha and -gamma receptors with IC(50) values of 0.98 and 0.092 microM, respectively. The lack of hPPARdelta activity could be explained by the absence of binding in the tail-up pocket in the hPPARdelta receptor, in contrast to the hPPARdelta agonist GW2433, which was able to bind in both the tail-up and tail-down pockets of the receptor.
AZ 242, a novel PPARalpha/gamma agonist with beneficial effects on insulin resistance and carbohydrate and lipid metabolism in ob/ob mice and obese Zucker rats.[Pubmed:12401884]
J Lipid Res. 2002 Nov;43(11):1855-63.
Abnormalities in fatty acid (FA) metabolism underlie the development of insulin resistance and alterations in glucose metabolism, features characteristic of the metabolic syndrome and type 2 diabetes that can result in an increased risk of cardiovascular disease. We present pharmacodynamic effects of AZ 242, a novel peroxisome proliferator activated receptor (PPAR)alpha/gamma agonist. AZ 242 dose-dependently reduced the hypertriglyceridemia, hyperinsulinemia, and hyperglycemia of ob/ob diabetic mice. Euglycemic hyperinsulinemic clamp studies showed that treatment with AZ 242 (1 micromol/kg/d) restored insulin sensitivity of obese Zucker rats and decreased insulin secretion. In vitro, in reporter gene assays, AZ 242 activated human PPARalpha and PPARgamma with EC(50) in the micro molar range. It also induced differentiation in 3T3-L1 cells, an established PPARgamma effect, and caused up-regulation of liver fatty acid binding protein in HepG-2 cells, a PPARalpha-mediated effect. PPARalpha-mediated effects of AZ 242 in vivo were documented by induction of hepatic cytochrome P 450-4A in mice. The results indicate that the dual PPARalpha/gamma agonism of AZ 242 reduces insulin resistance and has beneficial effects on FA and glucose metabolism. This effect profile could provide a suitable therapeutic approach to the treatment of type 2 diabetes, metabolic syndrome, and associated vascular risk factors.
Structure of the PPARalpha and -gamma ligand binding domain in complex with AZ 242; ligand selectivity and agonist activation in the PPAR family.[Pubmed:11587644]
Structure. 2001 Aug;9(8):699-706.
BACKGROUND: The peroxisome proliferator-activated receptors (PPAR) are ligand-activated transcription factors belonging to the nuclear receptor family. The roles of PPARalpha in fatty acid oxidation and PPARgamma in adipocyte differentiation and lipid storage have been characterized extensively. PPARs are activated by fatty acids and eicosanoids and are also targets for antidyslipidemic drugs, but the molecular interactions governing ligand selectivity for specific subtypes are unclear due to the lack of a PPARalpha ligand binding domain structure. RESULTS: We have solved the crystal structure of the PPARalpha ligand binding domain (LBD) in complex with the combined PPARalpha and -gamma agonist AZ 242, a novel dihydro cinnamate derivative that is structurally different from thiazolidinediones. In addition, we present the crystal structure of the PPARgamma_LBD/AZ 242 complex and provide a rationale for ligand selectivity toward the PPARalpha and -gamma subtypes. Heteronuclear NMR data on PPARalpha in both the apo form and in complex with AZ 242 shows an overall stabilization of the LBD upon agonist binding. A comparison of the novel PPARalpha/AZ 242 complex with the PPARgamma/AZ 242 complex and previously solved PPARgamma structures reveals a conserved hydrogen bonding network between agonists and the AF2 helix. CONCLUSIONS: The complex of PPARalpha and PPARgamma with the dual specificity agonist AZ 242 highlights the conserved interactions required for receptor activation. Together with the NMR data, this suggests a general model for ligand activation in the PPAR family. A comparison of the ligand binding sites reveals a molecular explanation for subtype selectivity and provides a basis for rational drug design.


