TaxinineCAS# 3835-52-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 3835-52-7 | SDF | Download SDF |
| PubChem ID | 10054413 | Appearance | Powder |
| Formula | C35H42O9 | M.Wt | 606.71 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
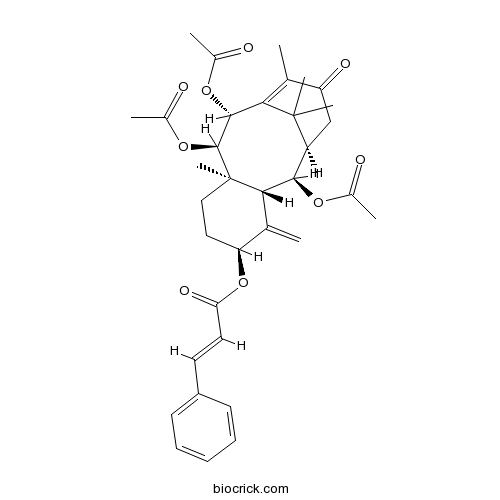
| SMILES | CC1=C2C(C(C3(CCC(C(=C)C3C(C(C2(C)C)CC1=O)OC(=O)C)OC(=O)C=CC4=CC=CC=C4)C)OC(=O)C)OC(=O)C | ||
| Standard InChIKey | BAYHEZUZRPMUDM-RZHPVIQDSA-N | ||
| Standard InChI | InChI=1S/C35H42O9/c1-19-26(39)18-25-31(41-21(3)36)30-20(2)27(44-28(40)15-14-24-12-10-9-11-13-24)16-17-35(30,8)33(43-23(5)38)32(42-22(4)37)29(19)34(25,6)7/h9-15,25,27,30-33H,2,16-18H2,1,3-8H3/b15-14+/t25-,27-,30-,31+,32+,33-,35+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Taxinine and Taxinine B can inhibit the drug transport by P-glycoprotein in multidrug-resistant cells. |
| Targets | P-gp |

Taxinine Dilution Calculator

Taxinine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6482 mL | 8.2412 mL | 16.4823 mL | 32.9647 mL | 41.2058 mL |
| 5 mM | 0.3296 mL | 1.6482 mL | 3.2965 mL | 6.5929 mL | 8.2412 mL |
| 10 mM | 0.1648 mL | 0.8241 mL | 1.6482 mL | 3.2965 mL | 4.1206 mL |
| 50 mM | 0.033 mL | 0.1648 mL | 0.3296 mL | 0.6593 mL | 0.8241 mL |
| 100 mM | 0.0165 mL | 0.0824 mL | 0.1648 mL | 0.3296 mL | 0.4121 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Neuropeptide W-23 (human)
Catalog No.:BCC5961
CAS No.:383415-79-0
- UC 112
Catalog No.:BCC8042
CAS No.:383392-66-3
- Homovanillic Acid Sulfate
Catalog No.:BCN2226
CAS No.:38339-06-9
- 3-O-beta-D-Glucopyranosylplatycodigenin
Catalog No.:BCN7832
CAS No.:38337-25-6
- YE 120
Catalog No.:BCC6188
CAS No.:383124-82-1
- Minoxidil
Catalog No.:BCC4297
CAS No.:38304-91-5
- Naringenin trimethyl ether
Catalog No.:BCN5437
CAS No.:38302-15-7
- Burchellin
Catalog No.:BCN6676
CAS No.:38276-59-4
- Glucose-conjugated MGMT inhibitor
Catalog No.:BCC1597
CAS No.:382607-78-5
- Malonomicin
Catalog No.:BCN1844
CAS No.:38249-71-7
- 20(R)-Ginsenoside Rg3
Catalog No.:BCN5018
CAS No.:38243-03-7
- beta-Amyrenonol
Catalog No.:BCN5436
CAS No.:38242-02-3
- 3',4'-Anhydrovinblastine
Catalog No.:BCN2392
CAS No.:38390-45-3
- NSC 663284
Catalog No.:BCC7199
CAS No.:383907-43-5
- Caudatin
Catalog No.:BCN5810
CAS No.:38395-02-7
- Ganaxolone
Catalog No.:BCC7397
CAS No.:38398-32-2
- Aloenin
Catalog No.:BCN8438
CAS No.:38412-46-3
- Altechromone A
Catalog No.:BCN7422
CAS No.:38412-47-4
- 3,4,4',7-Tetrahydroxyflavan
Catalog No.:BCN5438
CAS No.:38412-82-7
- Sclareol glycol
Catalog No.:BCN7007
CAS No.:38419-75-9
- H-Lys-OEt .2HCl
Catalog No.:BCC2980
CAS No.:3844-53-9
- Asatone
Catalog No.:BCN7761
CAS No.:38451-63-7
- Deacetyleupaserrin
Catalog No.:BCN7228
CAS No.:38456-39-2
- Eucannabinolide
Catalog No.:BCN7221
CAS No.:38458-58-1
A comparative optical aggregometry study of antiplatelet activity of taxanes from Taxus cuspidata.[Pubmed:20170941]
Thromb Res. 2010 Jun;125(6):e281-4.
Platelets are highly reactive components of the circulatory system. The cytoskeleton of a platelet is an important structure for platelet aggregation as stimulated by several agonists. An anticancer agent, taxol, has been suggested to exert platelet anti-aggregating activity by stabilizing microtubules during the aggregation process. An activity-guided fractionation was performed with a methanol extract of the leaves and twigs of Taxus cuspidata to isolate taxanes with platelet anti-aggregating effects. Compounds 1 to 7 - Taxinine (1), Taxinine A (2), Taxinine B (3), 2-deacetoxyTaxinine B (4), taxacin (5), taxchinin B (6), and taxol (7) - were obtained as the antiplatelet components of this plant. These taxane compounds present the possibility of securing new antiplatelet compounds which differ from currently available antiplatelet agents in chemical structure and possibly in mechanisms of action. All compounds showed stronger inhibitory effects than acetylsalicylic acid (ASA) on platelet aggregation induced by arachidonic acid (AA) (IC(50): 14.4, 64.5, 35.5, 16.0, 21.9, 28.6 and 48.2 versus 63.0microM) or U46619 (IC(50): 34.8, 24.9, 36.2, 35.0, 46.9, 71.9 and 68.7 versus 340microM). Compounds 1, 3, 4 and 5, with a cinnamoyl group at the C(5) position, showed strong inhibitory effects against AA-induced aggregation compared to compound 2 (with an -OH group at C(5)) or compounds with an oxetane ring at C(4),(5), such as compounds 6 and 7. All of the seven compounds were 5-13-fold more strongly inhibitory than ASA against U46619-induced aggregation.
Stabilization of microtubules by taxane diterpenoids: insight from docking and MD simulations.[Pubmed:25542396]
J Biol Phys. 2015 Mar;41(2):117-33.
Microtubules are formed from the molecules of tubulin, whose dynamics is important for many functions in a cell, the most dramatic of which is mitosis. Taxol is known to interact within a specific site on tubulin and also believed to block cell-cycle progression during mitosis by binding to and stabilizing microtubules. Along with the tremendous potential that taxol has shown as an anticancer drug, clinical problems exist with solubility, toxicity, and development of drug resistance. The crystal structure of taxane diterpenoids, namely, 10, 13-deacetyl-abeo-baccatin-IV (I), 5-acetyl-2-deacetoxydecinnamoyl-Taxinine-0.29hydrate (II), 7, 9-dideacetyltaxayuntin (III), and Taxawallin-K (IV), are very similar to the taxol molecule. Considerable attention has been given to such molecules whose archetype is taxol but do not posses long aliphatic chains, to be developed as a substitute for taxol with fewer side effects. In the present work, the molecular docking of these taxane diterpenoids has been carried out with the tubulin alpha-beta dimer (1TUB) and refined microtubule structure (1JFF) using Glide-XP, in order to assess the potential of tubulin binding of these cytotoxic agents. Results show that all the ligands dock into the classical taxol binding site of tubulin. Taxol shows the best binding capabilities. On the basis of docking energy and interactions, apart from taxol, molecule II has a better tendency of binding with 1TUB while molecule I shows better binding capability with bovine tubulin 1JFF. To validate the binding capabilities, molecular dynamics (MD) simulations of the best docked complexes of ligands with 1JFF have been carried out for 15.0 ns using DESMOND. Average RMSD variations and time line study of interactions and contacts indicate that these complexes remain stable during the course of the dynamics. However, taxol and molecule II prevail over other taxoids.
Simultaneous determination of seven taxoids in rat plasma by UPLC-MS/MS and pharmacokinetic study after oral administration of Taxus yunnanensis extracts.[Pubmed:25645339]
J Pharm Biomed Anal. 2015 Mar 25;107:346-54.
A rapid, sensitive and reliable method has been developed and validated for the simultaneous determination of seven taxoids including 10-deacetylbaccatin III (10-DAB III), baccatin III, 5-epi-canadensene, Taxinine M, 10-deacetyltaxol (10-DAT), cephalomannine and paclitaxel in rat plasma using docetaxel as the internal standard (IS). The plasma samples were pretreated by liquid-liquid extraction with methyl tert-butyl ether. The chromatographic separation was achieved on a C18 column (50 mm x 2.1 mm, 1.8 mum, Waters, USA) with a gradient elution program consisting of methanol and water (containing 0.1% formic acid) at a flow rate of 0.2 mL/min. Detection was performed under the selected reaction monitoring (SRM) scan using an electrospray ionization (ESI) in the positive ion mode. The mass transitions were as follows: m/z 567.4-->444.9 for 10-DAB III, m/z 609.0-->549.3 for baccatin III, m/z 617.4-->496.9 for 5-epi-canadensene, m/z 709.6-->649.3 for Taxinine M, m/z 834.8-->307.9 for 10-DAT, m/z 854.5-->285.4 for cephalomannine, m/z 876.8-->307.3 for paclitaxel and m/z 830.8-->549.6 for IS, respectively. All calibration curves exhibited good linearity (r(2)>0.99) over a wide concentration range for all components. The intra-day and inter-day precisions at three different levels were both less than 14.3% in terms of relative standard deviation (RSD) and the accuracies ranged from -8.3% to 14.8% in terms of relative error (RE). The extraction recoveries of the seven compounds ranged from 62.5% to 100.5%. The developed method was successfully applied to the pharmacokinetic study of the seven taxoids in rat plasma after oral administration of the crude extract of the twigs and leaves of Taxus yunnanensis.
Effect of seven tricyclic diterpenoids from needles of Taxus media var. Hicksii on stimulus-induced superoxide generation, tyrosyl or serine/threonine phosphorylation and translocation of cytosolic compounds to the cell membrane in human neutrophils.[Pubmed:19288401]
Planta Med. 2009 Jun;75(8):814-22.
Taxol has been widely used as an anticancer drug for ovarian, breast, lung and prostate cancer. Some kinds of Taxus plants are widely distributed in the Northeast Asia region. We have isolated seven tricyclic diterpenoids, Taxinine, taxagifine, 5-O-cinnamoyltaxacin I triacetate, 5-decinnamoylTaxinine J, 5-cinnamoyl-9-acetyltaxicin I, taxacin and taxol from the needles of Taxus media var. Hicksii, and investigated their effects on stimulus-induced superoxide generation and translocation of cytosolic compounds to the cell membrane in human neutrophils. Six tricyclic diterpenoids used in this experiment suppressed the superoxide generation induced by N-formyl-methionyl-leucyl-phenylalanine (fMLP) and arachidonic acid (AA) in a concentration-dependent manner. Taxinine significantly suppressed the superoxide generation induced by phorbol 12-myristate 13-acetate (PMA). The compounds also suppressed fMLP- and AA-induced tyrosyl or PMA-induced serine/threonine phosphorylation, and translocation of cytosolic compounds, p47 (phox), p67 (phox) and Rac to the cell membrane in parallel with the suppression of the stimulus-induced superoxide generation.


