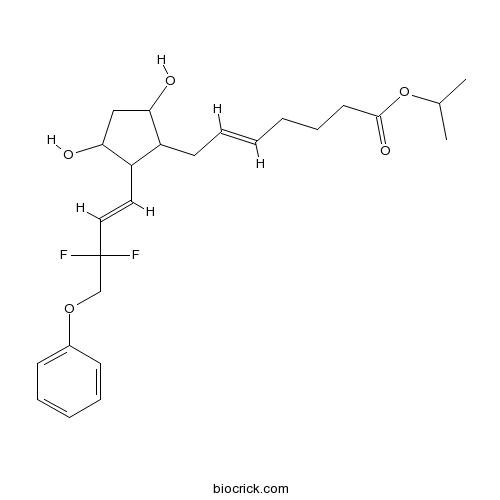
TafluprostCAS# 209860-87-7 |
- LDK378
Catalog No.:BCC3691
CAS No.:1032900-25-6
- AP26113
Catalog No.:BCC1069
CAS No.:1197958-12-5
- LDK378 dihydrochloride
Catalog No.:BCC1694
CAS No.:1380575-43-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 209860-87-7 | SDF | Download SDF |
| PubChem ID | 6433101 | Appearance | Powder |
| Formula | C25H34F2O5 | M.Wt | 452.53 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | AFP-168; MK2452 | ||
| Solubility | DMSO : ≥ 270 mg/mL (596.65 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | propan-2-yl (E)-7-[2-[(E)-3,3-difluoro-4-phenoxybut-1-enyl]-3,5-dihydroxycyclopentyl]hept-5-enoate | ||
| SMILES | CC(C)OC(=O)CCCC=CCC1C(CC(C1C=CC(COC2=CC=CC=C2)(F)F)O)O | ||
| Standard InChIKey | WSNODXPBBALQOF-ZHDUIJGQSA-N | ||
| Standard InChI | InChI=1S/C25H34F2O5/c1-18(2)32-24(30)13-9-4-3-8-12-20-21(23(29)16-22(20)28)14-15-25(26,27)17-31-19-10-6-5-7-11-19/h3,5-8,10-11,14-15,18,20-23,28-29H,4,9,12-13,16-17H2,1-2H3/b8-3+,15-14+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Tafluprost Dilution Calculator

Tafluprost Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2098 mL | 11.049 mL | 22.098 mL | 44.196 mL | 55.245 mL |
| 5 mM | 0.442 mL | 2.2098 mL | 4.4196 mL | 8.8392 mL | 11.049 mL |
| 10 mM | 0.221 mL | 1.1049 mL | 2.2098 mL | 4.4196 mL | 5.5245 mL |
| 50 mM | 0.0442 mL | 0.221 mL | 0.442 mL | 0.8839 mL | 1.1049 mL |
| 100 mM | 0.0221 mL | 0.1105 mL | 0.221 mL | 0.442 mL | 0.5524 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Tafluprost(AFP-168) is an anti-glaucoma prostaglandin (PG) analog. Target:Others Tafluprost showed significant IOP-lowering effects without any safety concerns in patients with various types of glaucoma and OH in daily clinical practice and tafluprost is highly effective in any therapeutic patterns [1]. Tafluprost with reduced BAK has potential as a superior antiglaucoma drug, not only for its IOP-lowering effect, but also for its good corneal safety profile [2]. Tafluprost single-use vials treatment was effective in reducing IOP over the 3 years of this study, but visual field performance worsened by 10.3%-13.8% in patients with normal-tension glaucoma. Safety was satisfactory [3].
References:
[1]. Kuwayama, Y. and A. Nomura, Prospective observational post-marketing study of tafluprost for glaucoma and ocular hypertension: short-term efficacy and safety. Adv Ther, 2014. 31(4): p. 461-71.
[2]. Kumagami, T., et al., Comparison of corneal safety and intraocular pressure-lowering effect of tafluprost ophthalmic solution with other prostaglandin ophthalmic solutions. J Ocul Pharmacol Ther, 2014. 30(4): p. 340-5.
[3]. Inoue, K., A. Tanaka, and G. Tomita, Effects of tafluprost treatment for 3 years in patients with normal-tension glaucoma. Clin Ophthalmol, 2013. 7: p. 1411-6.
- AR-M 1000390 hydrochloride
Catalog No.:BCC6143
CAS No.:209808-47-9
- Entinostat (MS-275,SNDX-275)
Catalog No.:BCC3595
CAS No.:209783-80-2
- Sodium Aescinate
Catalog No.:BCN6266
CAS No.:20977-05-3
- CART (55-102) (rat)
Catalog No.:BCC6006
CAS No.:209615-79-2
- Dihydroisotanshinone I
Catalog No.:BCN2308
CAS No.:20958-18-3
- Isotanshinone I
Catalog No.:BCN2500
CAS No.:20958-17-2
- Isotanshinone IIA
Catalog No.:BCN2501
CAS No.:20958-15-0
- SB 271046 hydrochloride
Catalog No.:BCC1924
CAS No.:209481-24-3
- SB271046
Catalog No.:BCC5057
CAS No.:209481-20-9
- 1-(2-Amino-5-chlorophenyl)-1-(trifluoromethyl)-3-cyclopropyl-2-propyn-1-ol
Catalog No.:BCC8404
CAS No.:209414-27-7
- VX-745
Catalog No.:BCC3966
CAS No.:209410-46-8
- Platycoside A
Catalog No.:BCN3241
CAS No.:209404-00-2
- Resibufagin
Catalog No.:BCN8230
CAS No.:20987-24-0
- H-Phenylglycinol
Catalog No.:BCC2713
CAS No.:20989-17-7
- Androst-5-ene-3β,17β-diol 3,17-diacetate
Catalog No.:BCC8823
CAS No.:2099-26-5
- YO-01027 (Dibenzazepine, DBZ)
Catalog No.:BCC2100
CAS No.:209984-56-5
- LY-411575
Catalog No.:BCC2101
CAS No.:209984-57-6
- LY-411575 isomer 1
Catalog No.:BCC5443
CAS No.:209984-58-7
- LY-900009
Catalog No.:BCC2103
CAS No.:209984-68-9
- Erythbidin A
Catalog No.:BCN6859
CAS No.:210050-83-2
- Nilgirine
Catalog No.:BCN2100
CAS No.:21009-05-2
- Jatrophane I
Catalog No.:BCN7658
CAS No.:210108-85-3
- Jatrophane 2
Catalog No.:BCN1502
CAS No.:210108-86-4
- Jatrophane 3
Catalog No.:BCN1501
CAS No.:210108-87-5
24-Hour Efficacy and Ocular Surface Health with Preservative-Free Tafluprost Alone and in Conjunction with Preservative-Free Dorzolamide/Timolol Fixed Combination in Open-Angle Glaucoma Patients Insufficiently Controlled with Preserved Latanoprost Monotherapy.[Pubmed:27913991]
Adv Ther. 2017 Jan;34(1):221-235.
INTRODUCTION: The aim of the present study was to evaluate the 24-h efficacy, tolerability, and ocular surface health with preservative-free (PF) Tafluprost and a PF triple drug regimen comprising Tafluprost and dorzolamide/timolol fixed combination (DTFC) in open-angle glaucoma patients who were insufficiently controlled with preserved branded or generic latanoprost monotherapy and who exhibited signs or symptoms of ocular surface disease (OSD). METHODS: Prospective, observer-masked, crossover, comparison. Eligible consecutive open-angle glaucoma patients were randomized to either PF Tafluprost or the triple PF regimen for 3 months. They were then crossed over to the opposite therapy for another 3 months. At the end of the latanoprost run-in period and after each PF treatment period, patients underwent habitual 24-h intraocular pressure (IOP) monitoring with Goldmann tonometry in the sitting position (at 10:00, 14:00, 18:00, and 22:00) and Perkins tonometry in the supine position (at 02:00 and 06:00). Tolerability and selected ocular surface parameters were evaluated at baseline and the end of each treatment period. RESULTS: Forty-three open-angle glaucoma patients completed the trial. Mean 24-h IOP on preserved latanoprost was 22.2 +/- 3.9 mmHg. Compared with latanoprost monotherapy, PF Tafluprost obtained a greater reduction in mean, peak, and fluctuation of 24-h IOP including the 02:00 and 06:00 time points (P < 0.05). With the exception of 24-h fluctuation, the triple PF regimen provided significantly lower IOP parameters than latanoprost or PF Tafluprost (P < 0.001). Finally, PF Tafluprost therapy displayed significantly improved tear film break-up times (6.7 vs 6.0 s), corneal staining (1.3 vs 2.2), and Schirmer I test results (9.1 vs 8.2 mm) compared with the preserved latanoprost baseline (all P < 0.01). The triple PF regimen demonstrated similar tear film break-up times (6.1 vs 6.0 s) and Schirmer I test results (8.2 vs 8.2 mm) to latanoprost, but revealed a significant improvement in the corneal stain test (1.7 vs 2.2; P < 0.001). CONCLUSIONS: In this trial PF Tafluprost therapy provided statistically greater 24-h efficacy and improved tolerability compared with preserved latanoprost. The combination of PF Tafluprost and PF dorzolamide/timolol fixed combination was statistically and clinically more efficacious than both monotherapies and demonstrated similar ocular surface characteristics to preserved latanoprost monotherapy. TRIAL REGISTRATION: ClinicalTrials.gov (NCT02802137). FUNDING: Santen.
Analysis of the effects of preservative-free tafluprost on the tear proteome.[Pubmed:27829990]
Am J Transl Res. 2016 Oct 15;8(10):4025-4039. eCollection 2016.
The purpose of the present study was to assess the ocular surface health status in primary open angle glaucoma (POAG) patients switching from topical application of preserved latanoprost (LT) to preservative-free Tafluprost (PFT) by tear proteomic monitoring. Tear fluid of POAG patients showing dry eye symptoms, using LT and switching to PFT as well as tear fluid of healthy controls has been examined. Tear proteome dynamics was monitored over 24 weeks in a first mass spectrometric explorative analysis in a small POAG patient cohort (N = 3). Longitudinal responses of candidate proteins as well as cytokines were comparatively analyzed by microarray in a larger cohort of POAG patients (N = 16) and healthy controls (N = 15). Clinical parameters including tear breakup time (TBUT) and basal Schirmer test (BST) were recorded. Distinct post-switch level alterations could be documented in POAG tear proteins (> 1000). Cellular leakage proteins, dry eye related candidates and cytokines showed predominantly level diminishment in POAG patients and approximation to the tear protein level of healthy controls in response to PFT. Tear proteins like pyruvate kinase isozymes M1/M2 or galectin 7 displayed linear tear film level decline in POAG patients (R(2)>/=0.9; P < 0.05) distinctly converging the healthy level. Proteomic outcome fit well with improved clinical parameters, TBUT and BST. In conclusion, tear proteomic alterations indicated ocular surface recovery regarding epithelia leakage and inflammation recession. Together with improved clinical parameters the study output proposes beneficial effects of PFT glaucoma therapy.
A Novel Convergent Synthesis of the Potent Antiglaucoma Agent Tafluprost.[Pubmed:28146132]
Molecules. 2017 Jan 31;22(2). pii: molecules22020217.
Tafluprost (AFP-168, 5) is a unique 15-deoxy-15,15-difluoro-16-phenoxy prostaglandin F2alpha (PGF2alpha) analog used as an efficacious ocular hypotensive agent in the treatment of glaucoma and ocular hypertension, as monotherapy, or as adjunctive therapy to beta-blockers. A novel convergent synthesis of 5 was developed employing Julia-Lythgoe olefination of the structurally advanced prostaglandin phenylsulfone 16, also successfully applied for manufacturing of pharmaceutical grade latanoprost (2), travoprost (3) and bimatoprost (4), with an aldehyde omega-chain synthon 17. The use of the same prostaglandin phenylsulfone 16, as a starting material in parallel syntheses of all commercially available antiglaucoma PGF2alpha analogs 2-5, significantly reduces manufacturing costs resulting from its synthesis on an industrial scale and development of technological documentation. Another key aspect of the route developed is deoxydifluorination of a trans-13,14-en-15-one 30 with Deoxo-Fluor. Subsequent hydrolysis of protecting groups and final esterification of acid 6 yielded Tafluprost (5). The main advantages are the preparation of high purity Tafluprost (5) and the application of comparatively cheap reagents. The preparation and identification of two other Tafluprost acid derivatives, Tafluprost methyl ester (32) and Tafluprost ethyl amide (33), are also described.
Comparison study of intraocular pressure reduction efficacy and safety between latanoprost and tafluprost in Japanese with normal-tension glaucoma.[Pubmed:27601879]
Clin Ophthalmol. 2016 Aug 24;10:1633-7.
PURPOSE: To evaluate and compare the intraocular pressure (IOP) reduction efficacy and safety between the ophthalmic solutions 0.005% latanoprost (Lat) and 0.0015% Tafluprost (Taf) in Japanese patients with normal-tension glaucoma (NTG). METHODS: In this randomized nonmasked study, we prospectively enrolled 30 Japanese NTG patients who had used Lat monotherapy for more than 4 weeks, and randomly divided them into the following two groups: 1) Lat-to-Taf group (LT group) and 2) Taf-to-Lat group (TL group). At the beginning of the study, both groups were switched from initial Lat to Lat or Taf for 12 weeks, and then switched over to the other drug (crossover) for 12 additional weeks. At 0, 4, 12, 16, and 24 weeks, we evaluated each patient's IOP, conjunctival injection, and corneal epitheliopathy score, and at 0, 12, and 24 weeks, we evaluated their eyelash changes and pigmentation of the eyelids and irises. RESULTS: The mean IOP of the LT group (15 eyes) was 10.5, 10.6, and 11.1 mmHg, at 0, 12, and 24 weeks, respectively, whereas that of the TL group (15 eyes) was 11.7, 11.1, and 10.5 mmHg at 0, 12, and 24 weeks, respectively. No significant differences were found between the two groups and in the intragroup comparisons. Moreover, no significant differences were found between Lat and Taf in regard to the conjunctival injection score and corneal epitheliopathy score. Eyelash changes and eyelid and iris pigmentation were similar in both groups. CONCLUSION: The findings of this study show that Lat and Taf have equivalent efficacy and safety in Japanese patients with NTG.


