Sylvestroside ICAS# 71431-22-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 71431-22-6 | SDF | Download SDF |
| PubChem ID | 101967019 | Appearance | Powder |
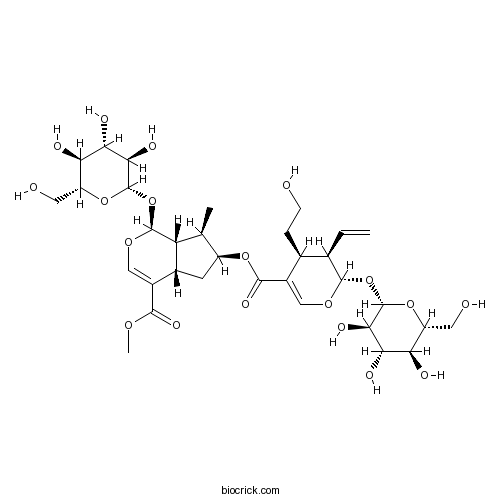
| Formula | C33H48O19 | M.Wt | 748.7 |
| Type of Compound | Iridoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | methyl (1S,4aS,6S,7R,7aS)-6-[(2S,3R,4S)-3-ethenyl-4-(2-hydroxyethyl)-2-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-3,4-dihydro-2H-pyran-5-carbonyl]oxy-7-methyl-1-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-1,4a,5,6,7,7a-hexahydrocyclopenta[c]pyran-4-carboxylate | ||
| SMILES | CC1C(CC2C1C(OC=C2C(=O)OC)OC3C(C(C(C(O3)CO)O)O)O)OC(=O)C4=COC(C(C4CCO)C=C)OC5C(C(C(C(O5)CO)O)O)O | ||
| Standard InChIKey | VSNATUGVSVGFFN-RXSNYNEVSA-N | ||
| Standard InChI | InChI=1S/C33H48O19/c1-4-13-14(5-6-34)16(10-46-30(13)51-32-26(41)24(39)22(37)19(8-35)49-32)29(44)48-18-7-15-17(28(43)45-3)11-47-31(21(15)12(18)2)52-33-27(42)25(40)23(38)20(9-36)50-33/h4,10-15,18-27,30-42H,1,5-9H2,2-3H3/t12-,13+,14-,15+,18-,19+,20+,21+,22+,23+,24-,25-,26+,27+,30-,31-,32-,33-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Sylvestroside I may have analgesic effect. |
| In vitro | Phytochemical and pharmacological studies on medicinal herb Acicarpha tribuloides.[Reference: WebLink]nternational Journal of Pharmacognosy,1996,34(4):255-61.
|

Sylvestroside I Dilution Calculator

Sylvestroside I Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.3356 mL | 6.6782 mL | 13.3565 mL | 26.713 mL | 33.3912 mL |
| 5 mM | 0.2671 mL | 1.3356 mL | 2.6713 mL | 5.3426 mL | 6.6782 mL |
| 10 mM | 0.1336 mL | 0.6678 mL | 1.3356 mL | 2.6713 mL | 3.3391 mL |
| 50 mM | 0.0267 mL | 0.1336 mL | 0.2671 mL | 0.5343 mL | 0.6678 mL |
| 100 mM | 0.0134 mL | 0.0668 mL | 0.1336 mL | 0.2671 mL | 0.3339 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Methylecgonine
Catalog No.:BCN1908
CAS No.:7143-09-1
- Plinabulin (NPI-2358)
Catalog No.:BCC5094
CAS No.:714272-27-2
- 3-Methyl-GABA
Catalog No.:BCC6629
CAS No.:71424-95-8
- Jatropholone B
Catalog No.:BCC8192
CAS No.:71386-38-4
- APETx2
Catalog No.:BCC6294
CAS No.:713544-47-9
- Mcl1-IN-1
Catalog No.:BCC5405
CAS No.:713492-66-1
- Moclobemide (Ro 111163)
Catalog No.:BCC2322
CAS No.:71320-77-9
- 6(1H)-Azulenone, 2,3-dihydro-1,4-dimethyl
Catalog No.:BCN1371
CAS No.:71305-89-0
- (S)-3-Hydroxyphenylglycine
Catalog No.:BCC6605
CAS No.:71301-82-1
- Cronaburmine
Catalog No.:BCN2072
CAS No.:71295-32-4
- Crotananine
Catalog No.:BCN2078
CAS No.:71295-28-8
- 2,5-Bis(5-tert-butyl-2-benzoxazolyl)thiophene
Catalog No.:BCC8503
CAS No.:7128-64-5
- TTNPB (Arotinoid Acid)
Catalog No.:BCC4874
CAS No.:71441-28-6
- Z-D-Asp(OtBu)-OH.H2O
Catalog No.:BCC2785
CAS No.:71449-08-6
- H-D-Val-OMe.HCl
Catalog No.:BCC3147
CAS No.:7146-15-8
- Vinorelbine
Catalog No.:BCN2543
CAS No.:71486-22-1
- AC480 (BMS-599626)
Catalog No.:BCC1252
CAS No.:714971-09-2
- Safrolglycol
Catalog No.:BCN4596
CAS No.:7154-01-0
- NSC 319726
Catalog No.:BCC2242
CAS No.:71555-25-4
- 3alpha,6beta-Ditigloyloxytropan-7beta-ol
Catalog No.:BCN1370
CAS No.:7159-86-6
- D-(-)-threo-2-Amino-1-(4-nitrophenyl)-1,3-propanediol
Catalog No.:BCC8924
CAS No.:716-61-0
- Biphenyl-3-carboxylic acid
Catalog No.:BCC8878
CAS No.:716-76-7
- Cephalomannine
Catalog No.:BCN5343
CAS No.:71610-00-9
- H-His-NH2.2HCl
Catalog No.:BCC2955
CAS No.:71666-95-0
Bis-iridoid and iridoid glycosides: Viral protein R inhibitors from Picrorhiza kurroa collected in Myanmar.[Pubmed:30794917]
Fitoterapia. 2019 Feb 19;134:101-107.
Four new bis-iridoid glycosides, saungmaygaosides A-D (1-4), and six known iridoid glycosides (5-10) were isolated from the n-butanol extract of the stems of Picrorhiza kurroa collected in Myanmar. Their structures were elucidated by extensive spectroscopic techniques. All of the isolates were assayed for anti-Vpr activity, using TREx-HeLa-Vpr cells. Among the isolates, saungmaygaoside D (4), Sylvestroside IV dimethyl acetal (7), and sweroside (8) were the most potent inhibitors with effective doses of 5 and 10muM, respectively, without showing any notable cytotoxicities.
A dimeric iridoid from loasa acerifolia.[Pubmed:11711085]
Phytochemistry. 1998 Nov 20;49(6):1705-1707.
Reinvestigation of leaf material from Loasa acerifolia DOMBEY led to the isolation of secoxyloganin and an additional novel dimeric iridoid glucoside named asaolaside. The latter consists of a secoxyloganin moiety esterified to the 7-hydroxy group of Sylvestroside IV. The structure of asaolaside was established by 1D and 2D NMR ((1)H (1)H COSY, HMQC, HMBC) and FABMS experiments.
Iridoids from Scaevola racemigera1.[Pubmed:17262339]
Planta Med. 1989 Apr;55(2):191-2.
Five iridoids have been isolated from the aerial parts of SCAEVOLA RACEMIGERA Daniker, namely, loganin, loganic acid, Sylvestroside III, cantleyoside, and scaevoloside. This latter is a novel compound whose structure 1 has been elucidated on the basis of its spectral data, mainly (1)H- and (13)C-NMR.
[Plants in New Caledonia. Iridoids from Scaevola montana Labill].[Pubmed:2637646]
Ann Pharm Fr. 1989;47(4):249-54.
Five iridoids have been isolated from the aerial parts of Scaevola montana Labill., namely loganin, Sylvestroside III, Sylvestroside III dimethylacetal, cantleyoside and cantleyoside dimethylacetal. Their structures have been elucidated on the basis of their spectral data, mainly chemical ionisation mass spectrometry and 1H-NMR spectroscopy.


