SulfaquinoxalineCAS# 59-40-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 59-40-5 | SDF | Download SDF |
| PubChem ID | 5338 | Appearance | Powder |
| Formula | C14H12N4O2S | M.Wt | 300.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
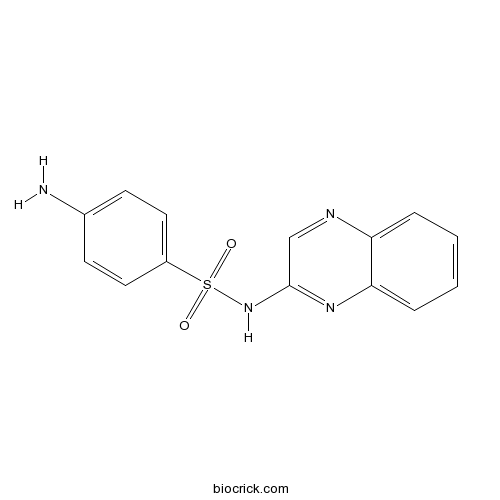
| Chemical Name | 4-amino-N-quinoxalin-2-ylbenzenesulfonamide | ||
| SMILES | C1=CC=C2C(=C1)N=CC(=N2)NS(=O)(=O)C3=CC=C(C=C3)N | ||
| Standard InChIKey | NHZLNPMOSADWGC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H12N4O2S/c15-10-5-7-11(8-6-10)21(19,20)18-14-9-16-12-3-1-2-4-13(12)17-14/h1-9H,15H2,(H,17,18) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Sulfaquinoxaline Dilution Calculator

Sulfaquinoxaline Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.33 mL | 16.65 mL | 33.3 mL | 66.6001 mL | 83.2501 mL |
| 5 mM | 0.666 mL | 3.33 mL | 6.66 mL | 13.32 mL | 16.65 mL |
| 10 mM | 0.333 mL | 1.665 mL | 3.33 mL | 6.66 mL | 8.325 mL |
| 50 mM | 0.0666 mL | 0.333 mL | 0.666 mL | 1.332 mL | 1.665 mL |
| 100 mM | 0.0333 mL | 0.1665 mL | 0.333 mL | 0.666 mL | 0.8325 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Mepyramine maleate
Catalog No.:BCC6740
CAS No.:59-33-6
- Folic acid
Catalog No.:BCN5375
CAS No.:59-30-3
- D-Galactose
Catalog No.:BCN8528
CAS No.:59-23-4
- 5-BrdU
Catalog No.:BCC5293
CAS No.:59-14-3
- Ethopabate
Catalog No.:BCC8964
CAS No.:59-06-3
- Methotrexate
Catalog No.:BCC2301
CAS No.:59-05-2
- DL-alpha-Tocopherol
Catalog No.:BCN2200
CAS No.:59-02-9
- Xanthurenic acid
Catalog No.:BCC7866
CAS No.:59-00-7
- Bestatin
Catalog No.:BCC1221
CAS No.:58970-76-6
- Cyclizine 2HCl
Catalog No.:BCC4518
CAS No.:5897-18-7
- D-Phe-Ol
Catalog No.:BCC2580
CAS No.:58917-85-4
- Z-Glu-OtBu
Catalog No.:BCC2778
CAS No.:5891-45-2
- Thiamine chloride
Catalog No.:BCN8344
CAS No.:59-43-8
- Procaine
Catalog No.:BCC5210
CAS No.:59-46-1
- Oxindole
Catalog No.:BCN4050
CAS No.:59-48-3
- DL-Methionine
Catalog No.:BCC8318
CAS No.:59-51-8
- Nicotinic acid
Catalog No.:BCN8328
CAS No.:59-67-6
- Nitrofurazone
Catalog No.:BCC3825
CAS No.:59-87-0
- Levodopa
Catalog No.:BCN1098
CAS No.:59-92-7
- Tolazoline HCl
Catalog No.:BCC4321
CAS No.:59-97-2
- Betaine hydrochloride
Catalog No.:BCN6304
CAS No.:590-46-5
- Bethanechol chloride
Catalog No.:BCC4566
CAS No.:590-63-6
- alpha-Endorphin
Catalog No.:BCC1010
CAS No.:59004-96-5
- 8-Hydroxyhyperforin 8,1-hemiacetal
Catalog No.:BCN4091
CAS No.:59014-02-7
Populations of Eimeria tenella express resistance to commonly used anticoccidial drugs in southern Nigeria.[Pubmed:30564595]
Int J Vet Sci Med. 2018 Jul 6;6(2):192-200.
Coccidiosis is one of the most economically important diseases of poultry. This study determined the preponderance of chicken Eimeria in southern Nigeria and assessed the parasite's resistance to three anticoccidial drugs: Amprolium hydrochloride; Amprolium hydrochloride+Sulfaquinoxaline-Sodium; and Toltrazuril. Multiplex PCR amplification of the SCAR region was used to confirm Eimeria preponderance. Resistance was assessed following the inoculation of 2.32x10(5) infective oocysts into broilers. Data on weight gain, feed intake, feed conversion and fecal oocyst shed were recorded. At 7days post inoculation 9 birds per treatment were sacrificed and assessed for macroscopic lesions in four intestinal regions. Percent optimum anticoccidial activity (POAA), Anticoccidial index (ACI) and Anticoccidial sensitivity test (AST) were used to access resistance. The preponderance of Eimeria spp. were E. tenella (77%), E. necatrix (55%), E. acervulina (44%) and E. mitis (11%), with multi-species infection occurring in 55% of samples assessed. Fecal oocyst shedding was low (P<0.05) in the medicated groups. Lesions in the cecal region were present in all infected groups regardless of treatment and accounted for 27.8% of lesion scores by severity and 37.5% of lesion scores by frequency. Overall, lesion scores were less (P<0.05) in birds of the medicated groups compared with the infected-unmedicated group. The high preponderance of E. tenella in the field, and the occurrence of cecal lesions - caused mainly by E. tenella- despite drug administration, indicate resistance in populations of this species in our isolate. Based-on the POAA, ACI and AST values, the Eimeria isolate showed reduced sensitivity to toltrazuril.
Transformation of sulfaquinoxaline by chlorine and UV light in water: kinetics and by-product identification.[Pubmed:29192403]
Environ Sci Pollut Res Int. 2018 Dec;25(35):34863-34872.
Sulfaquinoxaline (SQX) is an antimicrobial of the sulfonamide class, frequently detected at low levels in drinking and surface water as organic micropollutant. The main goal of the present study is the evaluation of SQX reactivity during chlorination and UV irradiations which are two processes mainly used in water treatment plants. The SQX transformation by chlorination and UV lights (254 nm) was investigated in purified water at common conditions used for water disinfection (pH = 7.2, temperature = 25 degrees C, [chlorine] = 3 mg L(-1)). The result shows a slow degradation of SQX during photolysis compared with chlorination process. Kinetic studies that fitted a fluence-based first-order kinetic model were used to determine the kinetic constants of SQX degradation; they were equal to 0.7 x 10(-4) and 0.7 x 10(-2) s(-1)corresponding to the half time lives of 162 and 1.64 min during photolysis and chlorination, respectively. In the second step, seven by-products were generated during a chlorination and photo-transformation of SQX and identified using liquid chromatography with electrospray ionization and tandem mass spectrometry (MS-MS). SO2 extrusion and direct decomposition were the common degradation pathway during photolysis and chlorination. Hydroxylation and isomerization were observed during photodegradation only while electrophilic substitution was observed during chlorination process.
Ferrous-activated peroxymonosulfate oxidation of antimicrobial agent sulfaquinoxaline and structurally related compounds in aqueous solution: kinetics, products, and transformation pathways.[Pubmed:28681293]
Environ Sci Pollut Res Int. 2017 Aug;24(24):19535-19545.
Sulfaquinoxaline (SQX) is a coccidiostatic drug widely used in poultry and swine production and has been frequently detected in various environmental compartments such as surface water, groundwater, soils, and sediments. In the present study, degradation of SQX by ferrous ion-activated peroxymonosulfate oxidation process (Fe(II)/PMS), a promising in situ chemical oxidation (ISCO) technique, was systematically investigated. Experimental results showed that Fe(II)/PMS process appeared to be more efficient for SQX removal relative to Fe(II)/persulfate process (Fe(II)/PS). An optimal Fe(II):PMS molar ratio of 1:1 was found to be necessary for efficient removal of SQX. Increasing the solution pH hampered the degradation of SQX, and no enhancement in SQX degradation was observed when chelating agents S,S'-ethylenediamine-N,N'-disuccinic acid (EDDS) and citrate were present. The presence of Suwannee River fulvic acid (SRFA), as a representative of aquatic natural organic matter (NOM), could inhibit the degradation of SQX. SQX was more susceptible to Fe(II)/PMS oxidation in comparison to its substructural analog 2-amino-quinoxaline (2-AQ) and other sulfonamides, i.e., sulfapyridine (SPD) and sulfadiazine (SDZ). Transformation products of SQX were enriched by solid-phase extraction (SPE) and identified by liquid chromatography-electrospray ionization-triple quadrupole mass spectrometry (LC-ESI-MS/MS). On the basis of the TPs identified, detailed reaction pathways for SQX degradation including sulfonamide bond cleavage, SO2 extrusion, and aniline moiety oxidation were proposed. Our contribution may provide some useful information for better understanding the kinetics and mechanisms of SQX degradation by sulfate radical-based advanced oxidation processes (SR-AOPs).


