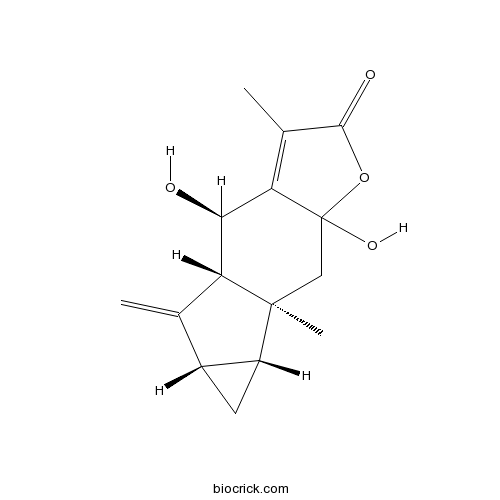
StrychnistenolideCAS# 332372-09-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 332372-09-5 | SDF | Download SDF |
| PubChem ID | 15484712 | Appearance | Powder |
| Formula | C15H18O4 | M.Wt | 262.30 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | CC1=C2C(C3C(=C)C4CC4C3(CC2(OC1=O)O)C)O | ||
| Standard InChIKey | CMOIXESTWPHDCC-MOGPIVFESA-N | ||
| Standard InChI | InChI=1S/C15H18O4/c1-6-8-4-9(8)14(3)5-15(18)11(12(16)10(6)14)7(2)13(17)19-15/h8-10,12,16,18H,1,4-5H2,2-3H3/t8-,9-,10-,12-,14+,15?/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| In vitro | Sesquiterpene lactones from the root tubers of Lindera aggregata.[Pubmed: 19639966 ]J Nat Prod. 2009 Aug;72(8):1497-501.
|
| Structure Identification | J Nat Prod. 2001 Mar;64(3):286-8.New eudesmane sesquiterpenes from the root of Lindera strychnifolia.[Pubmed: 11277740]
|

Strychnistenolide Dilution Calculator

Strychnistenolide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.8124 mL | 19.0621 mL | 38.1243 mL | 76.2486 mL | 95.3107 mL |
| 5 mM | 0.7625 mL | 3.8124 mL | 7.6249 mL | 15.2497 mL | 19.0621 mL |
| 10 mM | 0.3812 mL | 1.9062 mL | 3.8124 mL | 7.6249 mL | 9.5311 mL |
| 50 mM | 0.0762 mL | 0.3812 mL | 0.7625 mL | 1.525 mL | 1.9062 mL |
| 100 mM | 0.0381 mL | 0.1906 mL | 0.3812 mL | 0.7625 mL | 0.9531 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 1,7-Bis(4-hydroxyphenyl)hepta-4,6-dien-3-one
Catalog No.:BCN7092
CAS No.:332371-82-1
- Rutaevin
Catalog No.:BCN6993
CAS No.:33237-37-5
- DZ2002
Catalog No.:BCC5544
CAS No.:33231-14-0
- Glucosyringic acid
Catalog No.:BCN5254
CAS No.:33228-65-8
- TCS 5861528
Catalog No.:BCC7816
CAS No.:332117-28-9
- H-Ala-NH2.HCl
Catalog No.:BCC2688
CAS No.:33208-99-0
- Telatinib (BAY 57-9352)
Catalog No.:BCC3879
CAS No.:332012-40-5
- Nτ-Methyl-His-OH
Catalog No.:BCC2957
CAS No.:332-80-9
- I2906
Catalog No.:BCC1637
CAS No.:331963-29-2
- 2',4'-Dihydroxy-7-methoxy-8-prenylflavan
Catalog No.:BCN6844
CAS No.:331954-16-6
- 2-(N,N-Dimethylamino)acetophenone
Catalog No.:BCN1747
CAS No.:3319-03-7
- ZM 447439
Catalog No.:BCC2169
CAS No.:331771-20-1
- ML SA1
Catalog No.:BCC6276
CAS No.:332382-54-4
- 5-Aminofluorescein
Catalog No.:BCC8733
CAS No.:3326-34-9
- 5,6-Dihydroyangonin
Catalog No.:BCN3566
CAS No.:3328-60-7
- Diltiazem HCl
Catalog No.:BCC4901
CAS No.:33286-22-5
- Gummiferin
Catalog No.:BCN8381
CAS No.:33286-30-5
- Dipsacoside B
Catalog No.:BCN5940
CAS No.:33289-85-9
- Boc-Phe(4-NO2)-OH
Catalog No.:BCC3275
CAS No.:33305-77-0
- NBD-557
Catalog No.:BCC1791
CAS No.:333352-59-3
- NBD-556
Catalog No.:BCC1790
CAS No.:333353-44-9
- Trianthenol
Catalog No.:BCN7802
CAS No.:333361-85-6
- Gliquidone
Catalog No.:BCC5003
CAS No.:33342-05-1
- Theaflavine-3,3'-digallate
Catalog No.:BCN5420
CAS No.:33377-72-9
New eudesmane sesquiterpenes from the root of Lindera strychnifolia.[Pubmed:11277740]
J Nat Prod. 2001 Mar;64(3):286-8.
Strychnistenolide (1) and its acetate 2 were isolated from the root of Lindera strychnifolia, along with a novel rearranged type of secoeudesmane, strychnilactone (3). Their structures were elucidated by extensive analysis of their NMR spectra, including 2D NMR techniques, together with an X-ray analysis for 3. Strychnistenolide exists as a single stereoisomer in CHCl(3), but in pyridine is epimerized.
Sesquiterpene lactones from the root tubers of Lindera aggregata.[Pubmed:19639966]
J Nat Prod. 2009 Aug;72(8):1497-501.
Phytochemical investigation of the root tubers of Lindera aggregata resulted in the isolation of five new sesquiterpene lactones, linderagalactones A-E (1-5), along with eight known sesquiterpenoids, 3-eudesmene-1beta,11-diol, hydroxylindestenolide, Strychnistenolide, hydroxyisogermafurenolide, atractylenolide III, linderane, neolinderalactone, and linderalactone. The structures and relative configurations of 1-5 were determined by spectroscopic methods, especially HRESIMS and 2D NMR techniques. The absolute configurations of 1-4 were defined by comparison of quantum chemical TDDFT calculated and experimental ECD spectra. Linderagalactone A (1) is a halogenated sesquiterpene lactone possessing a unique rearranged carbon skeleton. Linderagalactone E (5), linderane, hydroxylindestenolide, and linderalactone showed hepatoprotective activity against H2O2-induced oxidative damages on HepG2 cells with EC(50) values of 67.5, 167.0, 42.4, and 98.0 microM, respectively.


