SparfloxacinCAS# 110871-86-8 |
- JNK-IN-7
Catalog No.:BCC1672
CAS No.:1408064-71-0
- CC-401 hydrochloride
Catalog No.:BCC1458
CAS No.:1438391-30-0
- CEP 1347
Catalog No.:BCC7982
CAS No.:156177-65-0
- AEG 3482
Catalog No.:BCC8088
CAS No.:63735-71-7
- AS 602801
Catalog No.:BCC1369
CAS No.:848344-36-5
- CC-930
Catalog No.:BCC1459
CAS No.:899805-25-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 110871-86-8 | SDF | Download SDF |
| PubChem ID | 60464 | Appearance | Powder |
| Formula | C19H22F2N4O3 | M.Wt | 392.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | 0.1 M NaOH : 50 mg/mL (127.42 mM; ultrasonic and adjust pH to 11 with NaOH) DMSO : 3.33 mg/mL (8.49 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
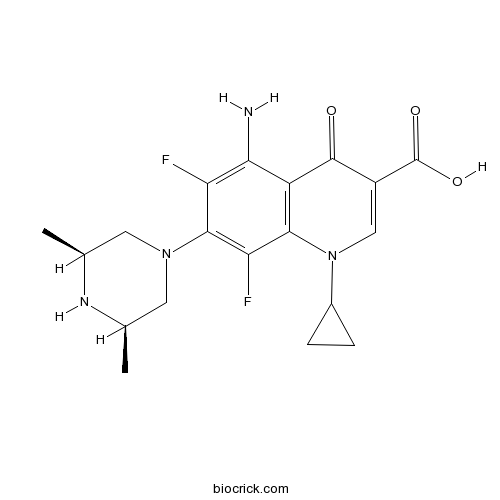
| Chemical Name | 5-amino-1-cyclopropyl-7-[(3S,5R)-3,5-dimethylpiperazin-1-yl]-6,8-difluoro-4-oxoquinoline-3-carboxylic acid | ||
| SMILES | C[C@H]1CN(C[C@@H](C)N1)c2c(F)c(N)c3C(=O)C(=CN(C4CC4)c3c2F)C(O)=O | ||
| Standard InChIKey | DZZWHBIBMUVIIW-DTORHVGOSA-N | ||
| Standard InChI | InChI=1S/C19H22F2N4O3/c1-8-5-24(6-9(2)23-8)17-13(20)15(22)12-16(14(17)21)25(10-3-4-10)7-11(18(12)26)19(27)28/h7-10,23H,3-6,22H2,1-2H3,(H,27,28)/t8-,9+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Sparfloxacin is a fluoroquinolone antibiotic, shows broad and potent antibacterial activity.
Target: Antibacterial
Sparfloxacin shows broad and potent antibacterial activity. Its MICs for 90% of the strains tested are 0.1 to 0.78 μg/ml against gram-positive organisms, such as members of the genera Staphylococcus , Streptococcus and Enterococcus , and 0.0125 to 1.56 μg/ml against gram-negative organisms, such as members of the family Enterobacteriaceae and the genera Pseudomona . Its MICs are 0.025 to 0.78 μg/ml against glucose nonfermenters, 0.2 to 0.78 μg/ml against anaerobes, 0.0125 to 0.05 μg/ml against Legionella. Sparfloxacin showed good oral efficacy against systemic infections with Staphylococcus aureus , Streptococcus pyogenes , Streptococcus pneumoniae , Escherichia coli , and Pseudomonas aeruginosa in mice [1]. Sparfloxacin targets DNA gyrase and inhibits DNA synthesis [2]. References: | |||||

Sparfloxacin Dilution Calculator

Sparfloxacin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5484 mL | 12.7421 mL | 25.4842 mL | 50.9684 mL | 63.7105 mL |
| 5 mM | 0.5097 mL | 2.5484 mL | 5.0968 mL | 10.1937 mL | 12.7421 mL |
| 10 mM | 0.2548 mL | 1.2742 mL | 2.5484 mL | 5.0968 mL | 6.371 mL |
| 50 mM | 0.051 mL | 0.2548 mL | 0.5097 mL | 1.0194 mL | 1.2742 mL |
| 100 mM | 0.0255 mL | 0.1274 mL | 0.2548 mL | 0.5097 mL | 0.6371 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Sparfloxacin is a fluoroquinolone antibiotic, shows broad and potent antibacterial activity.
- MCOPPB trihydrochloride
Catalog No.:BCC4161
CAS No.:1108147-88-1
- Cinobufotalin
Catalog No.:BCN2283
CAS No.:1108-68-5
- Garcinexanthone A
Catalog No.:BCN5993
CAS No.:1107620-67-6
- Asebotin
Catalog No.:BCN7233
CAS No.:11075-15-3
- beta-Escin
Catalog No.:BCC8172
CAS No.:11072-93-8
- Epimedin C
Catalog No.:BCN1040
CAS No.:110642-44-9
- Epimedin B
Catalog No.:BCN1039
CAS No.:110623-73-9
- Epimedin A
Catalog No.:BCN1038
CAS No.:110623-72-8
- human Insulin expressed in yeast
Catalog No.:BCC7689
CAS No.:11061-68-0
- Laminin (925-933)
Catalog No.:BCC1015
CAS No.:110590-60-8
- Salermide
Catalog No.:BCC7867
CAS No.:1105698-15-4
- Albrassitriol
Catalog No.:BCN7274
CAS No.:110557-39-6
- Entrectinib
Catalog No.:BCC6410
CAS No.:1108743-60-7
- Wilforine
Catalog No.:BCN5994
CAS No.:11088-09-8
- [Sar9,Met(O2)11]-Substance P
Catalog No.:BCC6960
CAS No.:110880-55-2
- Tunicamycin
Catalog No.:BCC7699
CAS No.:11089-65-9
- PF-04620110
Catalog No.:BCC2335
CAS No.:1109276-89-2
- Squalene
Catalog No.:BCN5995
CAS No.:111-02-4
- Decanedioic acid
Catalog No.:BCN5996
CAS No.:111-20-6
- Diethanolamine
Catalog No.:BCN1797
CAS No.:111-42-2
- Oleylethanolamide
Catalog No.:BCC7084
CAS No.:111-58-0
- 1-Heptylamine
Catalog No.:BCN1801
CAS No.:111-68-2
- Methyl Laurate
Catalog No.:BCC8211
CAS No.:111-82-0
- Deacetylsalannin
Catalog No.:BCN4733
CAS No.:1110-56-1
Crystal structures of [Mn(bdc)(Hspar)2(H2O)0.25].2H2O containing MnO6+1 capped trigonal prisms and [Cu(Hspar)2](bdc).2H2O containing CuO4 squares (Hspar = sparfloxacin and bdc = benzene-1,4-di-carboxyl-ate).[Pubmed:26870595]
Acta Crystallogr E Crystallogr Commun. 2016 Jan 1;72(Pt 1):96-101.
The syntheses and crystal structures of 0.25-aqua-(benzene-1,4-di-carboxyl-ato-kappa(2) O,O')bis-(Sparfloxacin-kappa(2) O,O')manganese(II) dihydrate, [Mn(C8H4O4)(C19H22F2N4O3)2(H2O)0.25].2H2O or [Mn(bdc)(Hspar)2(H2O)0.25].2H2O, (I), and bis-(Sparfloxacin-kappa(2) O,O')copper(II) benzene-1,4-di-carboxyl-ate dihydrate, [Cu(C19H22F2N4O3)2](C8H4O4).2H2O or [Cu(Hspar)2](bdc).2H2O, (II), are reported (Hspar = Sparfloxacin and bdc = benzene-1,4-di-carboxyl-ate). The Mn(2+) ion in (I) is coordinated by two O,O'-bidentate Hspar neutral mol-ecules (which exist as zwitterions) and an O,O'-bidentate bdc dianion to generate a distorted MnO6 trigonal prism. A very long bond [2.580 (12) A] from the Mn(2+) ion to a 0.25-occupied water mol-ecule projects through a square face of the prism. In (II), the Cu(2+) ion lies on a crystallographic inversion centre and a CuO4 square-planar geometry arises from its coordination by two O,O'-bidentate Hspar mol-ecules. The bdc dianion acts as a counter-ion to the cationic complex and does not bond to the metal ion. The Hspar ligands in both (I) and (II) feature intra-molecular N-Hcdots, three dots, centeredO hydrogen bonds, which close S(6) rings. In the crystals of both (I) and (II), the components are linked by N-Hcdots, three dots, centeredO, O-Hcdots, three dots, centeredO and C-Hcdots, three dots, centeredO hydrogen bonds, generating three-dimensional networks.
Cobalt(II) complexes of sparfloxacin: Characterization, structure, antimicrobial activity and interaction with DNA and albumins.[Pubmed:27501348]
J Inorg Biochem. 2016 Oct;163:18-27.
The cobalt(II) complexes with the quinolone Sparfloxacin (Hsf) in the absence or presence of the nitrogen-donor heterocyclic ligands 2,2'-bipyridine (bipy), 1,10-phenanthroline (phen) or 2,2'-bipyridylamine (bipyam) were prepared and characterized physicochemically and spectroscopically. The crystal structures of complexes [Co(sf)2(bipy)]3MeOH2H2O and [Co(sf)2(phen)]4MeOH were determined by X-ray crystallography. The antimicrobial activity of the complexes was tested against four different microorganisms (Escherichia coli, Xanthomonas campestris, Staphylococcus aureus and Bacillus subtilis) and was found similar or higher than that of free Hsf. The binding of the complexes to calf-thymus DNA was monitored by UV-vis spectroscopy and DNA-viscosity measurements and indirectly by competitive studies with ethidium bromide; intercalation is suggested as the most possible interaction mode. Fluorescence emission spectroscopy was used to evaluate the interaction of the complexes with human or bovine serum albumin and the corresponding binding constants were determined.
Comparison of the Antimicrobial Efficacy of Two Antibiotics Sparfloxacin and Augmentin as Experimental Root Canal Irrigating Solutions against Enterococcus faecalis - An Invitro Study.[Pubmed:27135003]
J Clin Diagn Res. 2016 Mar;10(3):ZC57-60.
INTRODUCTION: One of the main goals of endodontic treatment is root canal disinfection and to prevent subsequent chances of reinfection. Adjuvant to instrumentation, root canal irrigants are required to eliminate the bacteria found on the root canal walls and lateral canals within the dentinal tubules. AIM: To measure and compare the antibacterial efficacy of two antibiotics as experimental root canal irrigating solutions against Enterococcus faecalis (E. faecalis). MATERIALS AND METHODS: Fifteen Brain Heart Infusion agar plates were inoculated with Enterococcus faecalis-American Type Culture Collection (ATCC) 29212. 5 micrograms (mcg) Sparfloxacin discs, 30mcg Augmentin discs, and sterile paper test discs saturated with 2% Chlorhexidine (CHX), 3% Sodium Hypochlorite (NaOCl) and 5% NaOCl solutions were placed on agar plates. Sodium Chloride 0.9% (NaCl) paper discs were used as controls. Fifteen plates were incubated aerobically at 37 degrees C. Results were expressed as per the terms of the diameter of the inhibition zone. RESULTS: Results suggested a statistically significant difference in the zones of inhibition between five irrigating solutions (p < 0.001). CONCLUSION: Although, zones of inhibition were found in all the groups, 5mcg Sparfloxacin and 30mcg Augmentin showed maximum antimicrobial activity against E.faecalis.


