Sodium ascorbateCAS# 134-03-2 |
- Tiratricol
Catalog No.:BCC4738
CAS No.:51-24-1
Quality Control & MSDS
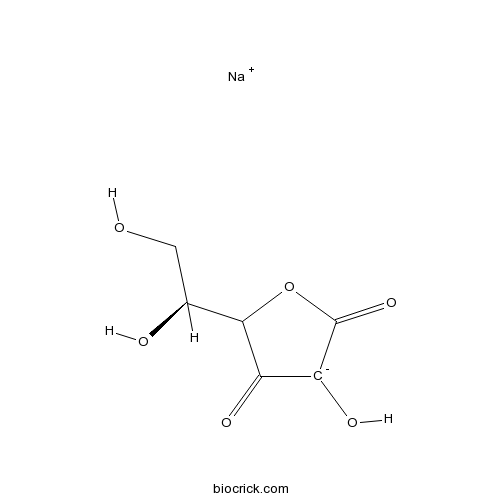
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 134-03-2 | SDF | Download SDF |
| PubChem ID | 45489831 | Appearance | Powder |
| Formula | C6H7NaO6 | M.Wt | 198.1 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | (+)-Sodium L-ascorbate; Vitamin C sodium salt; Sodium L-ascorbate | ||
| Solubility | H2O : 100 mg/mL (504.77 mM; Need ultrasonic) DMSO : 1 mg/mL (5.05 mM; Need ultrasonic) | ||
| Chemical Name | sodium;5-[(1S)-1,2-dihydroxyethyl]-3-hydroxyfuran-3-ide-2,4-dione | ||
| SMILES | C(C(C1C(=O)[C-](C(=O)O1)O)O)O.[Na+] | ||
| Standard InChIKey | IFVCRSPJFHGFCG-HXPAKLQESA-N | ||
| Standard InChI | InChI=1S/C6H7O6.Na/c7-1-2(8)5-3(9)4(10)6(11)12-5;/h2,5,7-8,10H,1H2;/q-1;+1/t2-,5?;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | L-Ascorbic acid (sodium) is a more bioavailable form of vitamin C that is an antioxidant agent.In Vitro:Vitamin C (L-ascorbic acid), a known enhancer of collagen deposition, has also been identified as an inhibitor of elastogenesis[1]. The conditioned medium for B16F10 cells significantly inhibits cell apoptosis induced by sodium L-ascorbate (10 mM), and the effective ingredients in the medium show a relative molecular mass below 5,000[2].In Vivo:Tg rats treated with sodium L-ascorbate show a higher incidence of carcinoma (29.6%), compared to those without sodium L-ascorbate (15.4%). Independent of the sodium L-ascorbate treatment, transgenic rats exhibit various kinds of malignant tumors in various organs[3]. After 12 weeks of PEITC-treatment, both simple hyperplasia and papillary or nodular (PN) hyperplasia have developed in all animals, but the majority of these lesions have disappeared at week 48, irrespective of the sodium L-ascorbate-treatment. The same lesions after 24 weeks of PEITC-treatment have progressed to dysplasia and carcinoma, in a small number of cases by week 48, but enhancement by the sodium L-ascorbate-treatment is evident only with simple hyperplasias and PN hyperplasias in rats[4]. References: | |||||

Sodium ascorbate Dilution Calculator

Sodium ascorbate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.048 mL | 25.2398 mL | 50.4796 mL | 100.9591 mL | 126.1989 mL |
| 5 mM | 1.0096 mL | 5.048 mL | 10.0959 mL | 20.1918 mL | 25.2398 mL |
| 10 mM | 0.5048 mL | 2.524 mL | 5.048 mL | 10.0959 mL | 12.6199 mL |
| 50 mM | 0.101 mL | 0.5048 mL | 1.0096 mL | 2.0192 mL | 2.524 mL |
| 100 mM | 0.0505 mL | 0.2524 mL | 0.5048 mL | 1.0096 mL | 1.262 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Sodium Ascorbate is a more bioavailable form of vitamin C that is an alternative to taking ascorbic acid as a supplement.
- Peonidin chloride
Catalog No.:BCN3016
CAS No.:134-01-0
- CUDC-907
Catalog No.:BCC2154
CAS No.:1339928-25-4
- Fmoc-Lys(2-Cl-Z)-OH
Catalog No.:BCC3513
CAS No.:133970-31-7
- Aristololactam I
Catalog No.:BCN2456
CAS No.:13395-02-3
- Rimantadine
Catalog No.:BCC4938
CAS No.:13392-28-4
- Kansuiphorin C
Catalog No.:BCN3764
CAS No.:133898-77-8
- ML224
Catalog No.:BCC5596
CAS No.:1338824-21-7
- Eicosyl ferulate
Catalog No.:BCN4712
CAS No.:133882-79-8
- Soyasaponin Ac
Catalog No.:BCN2897
CAS No.:133882-74-3
- Valnemulin HCl
Catalog No.:BCC4746
CAS No.:133868-46-9
- Safinamide
Catalog No.:BCC1915
CAS No.:133865-89-1
- OTS964
Catalog No.:BCC4025
CAS No.:1338545-07-5
- Pelargonidin chloride
Catalog No.:BCN3111
CAS No.:134-04-3
- Azaguanine-8
Catalog No.:BCC4629
CAS No.:134-58-7
- (-)-Lobeline hydrochloride
Catalog No.:BCC6927
CAS No.:134-63-4
- Lobeline Sulphate
Catalog No.:BCC8203
CAS No.:134-64-5
- 4-Hydroxy-3,5-dimethoxybenzaldehyde
Catalog No.:BCN6186
CAS No.:134-96-3
- d-Laserpitin
Catalog No.:BCN3616
CAS No.:134002-17-8
- Phaseollin
Catalog No.:BCN4816
CAS No.:13401-40-6
- Methyl beta-D-fructofuranoside
Catalog No.:BCN6183
CAS No.:13403-14-0
- H-Ala-OtBu.HCl
Catalog No.:BCC3194
CAS No.:13404-22-3
- Selaginellin F
Catalog No.:BCN6420
CAS No.:1340493-24-4
- (RS)-4-Carboxy-3-hydroxyphenylglycine
Catalog No.:BCC6598
CAS No.:134052-66-7
- (R)-4-Carboxyphenylglycine
Catalog No.:BCC6602
CAS No.:134052-68-9
Effect of sodium ascorbate and sodium nitrite on protein and lipid oxidation in dry fermented sausages.[Pubmed:27424306]
Meat Sci. 2016 Nov;121:359-364.
The effects of sodium nitrite and ascorbate on lipid and protein oxidation were studied during the ripening process of dry fermented sausages. Samples were taken at day 0, 2, 8, 14, 21 and 28 of ripening to assess lipid (malondialdehyde) and protein (carbonyls and sulfhydryl groups) oxidation. Sodium ascorbate and nitrite were separately able to reduce the formation of malondialdehyde. Their combined addition resulted in higher amounts of carbonyl compounds compared to their separate addition or the treatment without any of both compounds. Moreover, sodium nitrite limited the formation of gamma-glutamic semialdehyde whereas Sodium ascorbate showed a pro-oxidant effect. A loss of thiol groups was observed during ripening, which was not affected by the use of Sodium ascorbate nor sodium nitrite. In conclusion, sodium nitrite and ascorbate affected protein and lipid oxidation in different manners. The possible pro-oxidant effect of their combined addition on carbonyl formation might influence the technological and sensory properties of these products.
Photocatalytic ATRA reaction promoted by iodo-Bodipy and sodium ascorbate.[Pubmed:28085164]
Chem Commun (Camb). 2017 Feb 4;53(10):1591-1594.
Using ascorbate as a sacrificial reductant, iodo-Bodipy dye 1b is able to promote the ATRA reaction between bromoderivatives and alkenes. This finding expands the possibility of using Bodipy dyes to promote photocatalytic reactions in efficient ways.
Synergistic effects of sodium ascorbate and acetone to restore compromised bond strength after enamel bleaching.[Pubmed:28117857]
Int J Esthet Dent. 2017;12(1):86-94.
AIM: To evaluate the effect of a new experimental solution containing Sodium ascorbate (SA) and acetone on reversing compromised bonding to enamel immediately after bleaching. MATERIALS AND METHODS: The buccal surface of intact, extracted human premolars (n=60) was bleached. The teeth were then randomly assigned to 6 groups according to the type of pretreatment applied prior to adhesive procedures: 10% SA in acetone-water solution applied for 1 and 5 min (groups 1 and 2, respectively); aqueous solution of 10% SA applied for 10 min (group 3); 100% acetone applied for 10 min (group 4); no pretreatment (negative control; group 5). An additional group (positive control; group 6) comprised unbleached teeth (n=12). Two composite microcylinders were bonded on each specimen for evaluation of microshear bond strength (MBS) and failure modes. Data were analyzed using the one-way ANOVA and Tukey's post-hoc and chi-square tests at P=0.05. RESULTS: Groups 1 and 2 yielded similar MBS values to groups 4 and 6 (positive control). The mean MBS of groups 3 and 5 (negative control) were similar, and significantly lower than that of the positive control group. CONCLUSION: The application of 10% SA in an acetone-water solution prior to bonding procedures can restore compromised enamel bond strength to its unbleached state within a clinically acceptable time of 1 min.
Sodium ascorbate kills Candida albicans in vitro via iron-catalyzed Fenton reaction: importance of oxygenation and metabolism.[Pubmed:27855492]
Future Microbiol. 2016 Dec;11:1535-1547.
AIM: Ascorbate can inhibit growth and even decrease viability of various microbial species including Candida albicans. However the optimum conditions and the mechanism of action are unclear. Materials/methodology: Candida albicans shaken for 90 min in a buffered solution of ascorbate (90 mM) gave a 5-log reduction of cell viability, while there was no killing without shaking, in growth media with different carbon sources or at 4 degrees C. Killing was inhibited by the iron chelator 2,2'-bipyridyl. Hydroxyphenyl fluorescein probe showed the intracellular generation of hydroxyl radicals. RESULTS/CONCLUSION: Ascorbate-mediated killing of C. albicans depends on oxygenation and metabolism, involves iron-catalyzed generation of hydroxyl radicals via Fenton reaction and depletion of intracellular NADH. Ascorbate could serve as a component of a topical antifungal therapy.


