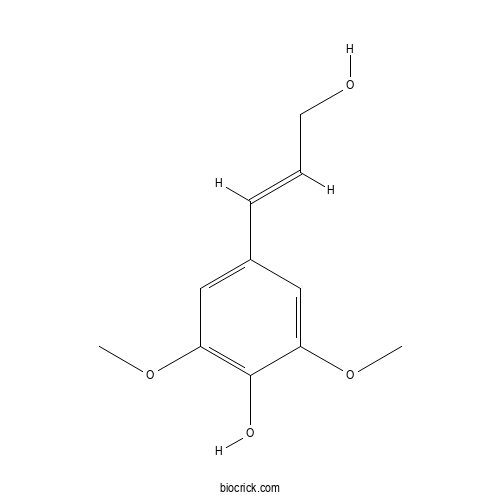
Sinapyl alcoholCAS# 537-33-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 537-33-7 | SDF | Download SDF |
| PubChem ID | 5280507 | Appearance | Powder |
| Formula | C11H14O4 | M.Wt | 210.23 |
| Type of Compound | Phenylpropanoid | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 4-[(E)-3-hydroxyprop-1-enyl]-2,6-dimethoxyphenol | ||
| SMILES | COC1=CC(=CC(=C1O)OC)C=CCO | ||
| Standard InChIKey | LZFOPEXOUVTGJS-ONEGZZNKSA-N | ||
| Standard InChI | InChI=1S/C11H14O4/c1-14-9-6-8(4-3-5-12)7-10(15-2)11(9)13/h3-4,6-7,12-13H,5H2,1-2H3/b4-3+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Sinapyl alcohol Dilution Calculator

Sinapyl alcohol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.7567 mL | 23.7835 mL | 47.567 mL | 95.1339 mL | 118.9174 mL |
| 5 mM | 0.9513 mL | 4.7567 mL | 9.5134 mL | 19.0268 mL | 23.7835 mL |
| 10 mM | 0.4757 mL | 2.3783 mL | 4.7567 mL | 9.5134 mL | 11.8917 mL |
| 50 mM | 0.0951 mL | 0.4757 mL | 0.9513 mL | 1.9027 mL | 2.3783 mL |
| 100 mM | 0.0476 mL | 0.2378 mL | 0.4757 mL | 0.9513 mL | 1.1892 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Rankinidine
Catalog No.:BCX0476
CAS No.:106466-66-4
- Methyl indole-3-acetate
Catalog No.:BCX0475
CAS No.:1912-33-0
- Galanal B
Catalog No.:BCX0474
CAS No.:104113-52-2
- Labda-8(17),12E,14-trien-16,15-olide
Catalog No.:BCX0473
CAS No.:917078-10-5
- Mulberrofuran V
Catalog No.:BCX0472
CAS No.:174423-49-5
- 6'-O-p-Hydroxybenzoylgastrodin
Catalog No.:BCX0471
CAS No.:1551525-70-2
- 15-Hydroxylabda-8(17),12E-dien-16-al
Catalog No.:BCX0470
CAS No.:283614-59-5
- Sanggenol F
Catalog No.:BCX0469
CAS No.:202526-51-0
- Moracin B
Catalog No.:BCX0468
CAS No.:67259-16-9
- Procumboside A
Catalog No.:BCX0467
CAS No.:850894-16-5
- Zataroside A
Catalog No.:BCX0466
CAS No.:95645-53-7
- 6β-Hydroxy-7-epi-α-cyperone
Catalog No.:BCX0465
CAS No.:6851-55-4
- Gypenoside LXXV
Catalog No.:BCX0478
CAS No.:110261-98-8
- Indole-3-acetic acid β-D-glucopyranosyl ester
Catalog No.:BCX0479
CAS No.:19817-95-9
- Schisanhenol B
Catalog No.:BCX0480
CAS No.:102681-52-7
- Schisandrathera D
Catalog No.:BCX0481
CAS No.:2694046-04-1
- Dencichine
Catalog No.:BCX0482
CAS No.:5302-45-4
- Deoxylimonin
Catalog No.:BCX0483
CAS No.:989-23-1
- 5-Methoxydadahol A
Catalog No.:BCX0484
CAS No.:2410566-84-4
- 4-(3-Hydroxydecyl)phenol
Catalog No.:BCX0485
CAS No.:1465124-36-0
- Oxyphyllone D
Catalog No.:BCX0486
CAS No.:1190094-25-7
- Rugulolide A
Catalog No.:BCX0487
CAS No.:3002032-70-1
- Rugulolide B
Catalog No.:BCX0488
CAS No.:3002032-71-2
- 2,4-Dihydroxybezaldehyde
Catalog No.:BCX0489
CAS No.:95-01-2
Metabolic changes in response to varying whole-grain wheat and rye intake.[Pubmed:38291073]
NPJ Sci Food. 2024 Jan 30;8(1):8.
Epidemiological studies have shown associations between whole-grain intake and lowered disease risk. A sufficient level of whole-grain intake to reach the health benefits has not been established, and there is limited knowledge about the impact of whole-grain intake on metabolite levels. In this clinical intervention study, we aimed to identify plasma and urine metabolites associated with two different intake levels of whole-grain wheat and rye and to correlate them with clinical plasma biomarkers. Healthy volunteers (N = 68) were divided into two groups receiving either whole-grain wheat or whole-grain rye in two four-week interventions with 48 and 96 g/d of whole grains consumed. The metabolomics of the plasma samples was performed with UPLC-QTOF-MS. Plasma alkylresorcinols were quantified with GC-MS and plasma and urinary mammalian lignans with HPLC-ECD. The high-dose intervention impacted the metabolite profile, including microbial metabolites, more in the rye-enriched diet compared with wheat. Among the increased metabolites were alkylresorcinol glucuronides, Sinapyl alcohol, and pipecolic acid betaine, while the decreased metabolites included acylcarnitines and ether lipids. Plasma alkylresorcinols, urinary enterolactone, and total mammalian lignans reflected the study diets in a dose-dependent manner. Several key metabolites linked with whole-grain consumption and gut microbial metabolism increased in a linear manner between the two interventions. The results reveal that an increase in whole-grain intake, particularly rye, is strongly reflected in the metabolite profile, is correlated with clinical variables, and suggests that a diet rich in whole grains promotes the growth and/or metabolism of microbes producing potentially beneficial microbial metabolites.
Identification and Functional Characterization of a Novel Sinapyl Alcohol Acyltransferase from Euphorbia lathyris L.[Pubmed:38044624]
J Agric Food Chem. 2023 Dec 20;71(50):20187-20197.
Methoxyeugenol is a phenylpropene compound derived from plants and has various bioactivities. The chemical synthesis of methoxyeugenol is accompanied by pollution issues, whereas extraction from plants is associated with problems such as low yield and high cost. The production of methoxyeugenol can be effectively addressed through an enzymatic approach. In this study, the acyltransferase genes of Euphorbia lathyris L. were screened by homologous alignment of the transcriptome data of E. lathyris in the late growth stage and the acyltransferase genes of the closely related plant species. The results showed that ElBAHD10 had the closest relationship with earlier reported ScCFAT and PhCFAT, which were found to catalyze the reaction of coniferyl alcohol to generate coniferyl acetate. The ElBAHD10 gene was successfully cloned from E. lathyris and subsequently expressed in Escherichia coli. The purified protein ElBAHD10 catalyzed the reaction of Sinapyl alcohol with acetyl CoA and cinnamoyl CoA to form sinapyl acetate and sinapyl cinnamate, respectively. In contrast, the crude ElBAHD10 protein could catalyze Sinapyl alcohol to directly generate methoxyeugenol. The recombinant E. coli strain expressing ElBAHD10 produced methoxyeugenol through whole-cell transformation. This study provides insights and lays the foundation for methoxyeugenol production through biosynthetic approaches.
One-Pot Biocatalytic Synthesis of rac-Syringaresinol from a Lignin-Derived Phenol.[Pubmed:38026814]
ACS Catal. 2023 Oct 31;13(22):14639-14649.
The drive for a circular bioeconomy has resulted in a great demand for renewable, biobased chemicals. We present a one-pot biocatalytic cascade reaction for the production of racemic syringaresinol, a lignan with applications as a nutraceutical and in polymer chemistry. The process consumes dihydroSinapyl alcohol, which can be produced renewably from the lignocellulosic material. To achieve this, a variant of eugenol oxidase was engineered for the oxidation of dihydroSinapyl alcohol into Sinapyl alcohol with good conversion and chemoselectivity. The crystal structure of the engineered oxidase revealed the molecular basis of the influence of the mutations on the chemoselectivity of the oxidation of dihydroSinapyl alcohol. By using horseradish peroxidase, the subsequent oxidative dimerization of Sinapyl alcohol into syringaresinol was achieved. Conditions for the one-pot, two-enzyme synthesis were optimized, and a high yield of syringaresinol was achieved by cascading the oxidase and peroxidase steps in a stepwise fashion. This study demonstrates the efficient production of syringaresinol from a compound that can be renewed by reductive catalytic fractionation of lignocellulose, providing a biocatalytic route for generating a valuable compound from lignin.
Corn straw lignin - A sustainable bioinspired finish for superhydrophobic and UV-protective cellulose fabric.[Pubmed:38013073]
Int J Biol Macromol. 2024 Feb;257(Pt 1):128393.
Hydrophobic textiles have been considered extensively for self-cleaning, phase-separating, and biomedical curing applications. We focused on preparing an eco-friendly lignin-based bio-finish to develop superhydrophobic cellulose fabric under mild conditions. The mass spectroscopic analysis expressed that the lignin comprised the major constituents of p-coumaryl alcohol, ferulic acid, coniferyl alcohol, and Sinapyl alcohol. The surface morphological analysis indicated the formation of a regular lignin coating on the cellulose fabric. The bio-finished cellulose fabric prepared (at 2 %, w/v, lignin) expressed the maximum water contact angle (WCA) of 157.2 degrees and remained in the hydrophobic range (119 degrees ) after ten standard washes. The treated fabric expressed the WCA values of 135.0 and 133.0 degrees after exposure to pH 2 and 12 aqueous media, respectively. The infrared spectroscopic analysis indicated the functional chemistry of the precursors involved and possible alteration in their chemical interactions during processing. The lignin-treated cellulose was observed to be less crystalline as compared to the untreated one. Such fabric expressed acceptable comfort, sensorial properties, and thermal stability up to 333 degrees C. The treated fabrics could block up to 92.24 % UV-A and 98.62 % UV-B radiations. Consequently, the lignin-based finish sourced from wasted corn straw was found cost-effective and efficient for producing superhydrophobic cellulose fabric.
Silicon inhibits cadmium uptake by regulating the genes associated with the lignin biosynthetic pathway and plant hormone signal transduction in maize plants.[Pubmed:37995035]
Environ Sci Pollut Res Int. 2023 Dec;30(59):123996-124009.
Cadmium (Cd) contamination in soil poses a severe threat to plant growth and development. In contrast, silicon (Si) has shown promise in enhancing plant resilience under Cd-induced stress. In this study, we conducted an integrated investigation employing morphological studies, gene expression analysis, and metabolomics to unravel the molecular mechanisms underlying Cd tolerance in maize plants. Our results demonstrate that Si biofortification significantly mitigated Cd stress by reducing Cd accumulation in plant tissues, increasing Si content, and enhancing maize biomass in Cd-stressed plants resulted in a substantial enhancement in shoot dry weight (+ 75%) and root dry weight (+ 30%). Notably, Si treatment upregulated key lignin-related genes (TaPAL, TaCAD, Ta4CL, and TaCOMT) and promoted the accumulation of metabolites (Sinapyl alcohol, phenylalanine, p-coumaryl alcohol, cafeyl alcohol, and coniferaldehyde) essential for cell wall strength, particularly under Cd stress conditions. Si application enriched the signal transduction by hormones and increased resistance by induction of biosynthesis genes (TaBZR1, TaLOX3, and TaNCDE1) and metabolites (brassinolide, abscisic acid, and jasmonate) in the roots and leaves under Cd stress. Furthermore, our study provides a comprehensive view of the intricate molecular crosstalk between Si, Cd stress, and plant hormonal responses. We unveil a network of genetic and metabolic interactions that culminate in a multifaceted defense system, enabling maize plants to thrive even in the presence of Cd-contaminated soil. This knowledge not only advances our understanding of the protective role of Si but also highlights the broader implications for sustainable agricultural practices. By harnessing the insights gained from this research, we may pave the way for innovative strategies to fortify crops against environmental stressors, ultimately contributing to the goal of food security in an ever-changing world. In summary, our research offers valuable insights into the protective mechanisms facilitated by Si, which enhance plants' ability to withstand environmental stress, and holds promise for future applications in sustainable agriculture.
Carboxylic acid reductase-dependent biosynthesis of eugenol and related allylphenols.[Pubmed:37980525]
Microb Cell Fact. 2023 Nov 18;22(1):238.
BACKGROUND: (Hydroxy)cinnamyl alcohols and allylphenols, including coniferyl alcohol and eugenol, are naturally occurring aromatic compounds widely utilised in pharmaceuticals, flavours, and fragrances. Traditionally, the heterologous biosynthesis of (hydroxy)cinnamyl alcohols from (hydroxy)cinnamic acids involved CoA-dependent activation of the substrate. However, a recently explored alternative pathway involving carboxylic acid reductase (CAR) has proven efficient in generating the (hydroxy)cinnamyl aldehyde intermediate without the need for CoA activation. In this study, we investigated the application of the CAR pathway for whole-cell bioconversion of a range of (hydroxy)cinnamic acids into their corresponding (hydroxy)cinnamyl alcohols. Furthermore, we sought to extend the pathway to enable the production of a variety of allylphenols and allylbenzene. RESULTS: By screening the activity of several heterologously expressed enzymes in crude cell lysates, we identified the combination of Segniliparus rugosus CAR (SrCAR) and Medicago sativa cinnamyl alcohol dehydrogenase (MsCAD2) as the most efficient enzymatic cascade for the two-step reduction of ferulic acid to coniferyl alcohol. To optimise the whole-cell bioconversion in Escherichia coli, we implemented a combinatorial approach to balance the gene expression levels of SrCAR and MsCAD2. This optimisation resulted in a coniferyl alcohol yield of almost 100%. Furthermore, we extended the pathway by incorporating coniferyl alcohol acyltransferase and eugenol synthase, which allowed for the production of eugenol with a titre of up to 1.61 mM (264 mg/L) from 3 mM ferulic acid. This improvement in titre surpasses previous achievements in the field employing a CoA-dependent coniferyl alcohol biosynthesis pathway. Our study not only demonstrated the successful utilisation of the CAR pathway for the biosynthesis of diverse (hydroxy)cinnamyl alcohols, such as p-coumaryl alcohol, caffeyl alcohol, cinnamyl alcohol, and Sinapyl alcohol, from their corresponding (hydroxy)cinnamic acid precursors but also extended the pathway to produce allylphenols, including chavicol, hydroxychavicol, and methoxyeugenol. Notably, the microbial production of methoxyeugenol from sinapic acid represents a novel achievement. CONCLUSION: The combination of SrCAR and MsCAD2 enzymes offers an efficient enzymatic cascade for the production of a wide array of (hydroxy)cinnamyl alcohols and, ultimately, allylphenols from their respective (hydroxy)cinnamic acids. This expands the range of value-added molecules that can be generated using microbial cell factories and creates new possibilities for applications in industries such as pharmaceuticals, flavours, and fragrances. These findings underscore the versatility of the CAR pathway, emphasising its potential in various biotechnological applications.
Root Exposure of Graphitic Carbon Nitride (g-C(3)N(4)) Modulates Metabolite Profile and Endophytic Bacterial Community to Alleviate Cadmium- and Arsenate-Induced Phytotoxicity to Rice (Oryza sativa L.).[Pubmed:37812587]
ACS Nano. 2023 Oct 24;17(20):19724-19739.
To investigate the mechanisms by which g-C(3)N(4) alleviates metal(loid)-induced phytotoxicity, rice seedlings were exposed to 100 and 250 mg/kg graphitic carbon nitride (g-C(3)N(4)) with or without coexposure to 10 mg/kg Cd and 50 mg/kg As for 30 days. Treatment with 250 mg/kg g-C(3)N(4) significantly increased shoot and root fresh weight by 22.4-29.9%, reduced Cd and As accumulations in rice tissues by 20.6-26.6%, and elevated the content of essential nutrients (e.g., K, S, Mg, Cu, and Zn) compared to untreated controls. High-throughput sequencing showed that g-C(3)N(4) treatment increased the proportion of plant-growth-promoting endophytic bacteria, including Streptomyces, Saccharimonadales, and Thermosporothrix, by 0.5-3.30-fold; these groups are known to be important to plant nutrient assimilation, as well as metal(loid) resistance and bioremediation. In addition, the population of Deinococcus was decreased by 72.3%; this genus is known to induce biotransformation As(V) to As(III). Metabolomics analyses highlighted differentially expressed metabolites (DEMs) involved in the metabolism of tyrosine metabolism, pyrimidines, and purines, as well as phenylpropanoid biosynthesis related to Cd/As-induced phytotoxicity. In the phenylpropanoid biosynthesis pathway, the increased expression of 4-coumarate (1.13-fold) and Sinapyl alcohol (1.26-fold) triggered by g-C(3)N(4) coexposure with Cd or As played a critical role in promoting plant growth and enhancing rice resistance against metal(loid) stresses. Our findings demonstrate the potential of g-C(3)N(4) to enhance plant growth and minimize the Cd/As-induced toxicity in rice and provide a promising nanoenabled strategy for remediating heavy metal(loid)-contaminated soil.
Initial Stage of Syringyl Lignin Formation from Sinapyl Alcohol.[Pubmed:37774119]
J Agric Food Chem. 2023 Oct 11;71(40):14666-14677.
Elucidating the detailed structure and formation mechanism of lignin, especially understudied syringyl (S) lignin, advances our knowledge of lignocellulosic biomass. To examine the early stages of S-lignin formation from Sinapyl alcohol (SA), the FMR (flow microreactor) method and the Zutropf (gradual addition of SA) method with limited amounts of H(2)O(2) were employed for the peroxidase-catalyzed dehydrogenative polymerization of SA. Only beta-beta dimers and not beta-O-4 dimers were obtained as initial dimerization products. Six new oligoligognols up to pentamers with beta-beta and beta-O-4 structures were identified. The erythro isomer was preferentially formed over the threo isomer in the beta-O-4 structures, similar to that found in naturally occurring S-rich hardwood lignin. Although minor substructures, the alpha-oxidized beta-beta and beta-O-4 structures and spirodienone (beta-1) structure identified in this study demonstrate the characteristic features of S-rich lignin. Based on the identified products, the initial formation mechanism of S-lignin from SA was proposed.
Hydroxycinnamaldehyde-derived benzofuran components in lignins.[Pubmed:37773018]
Plant Physiol. 2024 Feb 29;194(3):1370-1382.
Lignin is an abundant polymer in plant secondary cell walls. Prototypical lignins derive from the polymerization of monolignols (hydroxycinnamyl alcohols), mainly coniferyl and Sinapyl alcohol, via combinatorial radical coupling reactions and primarily via the endwise coupling of a monomer with the phenolic end of the growing polymer. Hydroxycinnamaldehyde units have long been recognized as minor components of lignins. In plants deficient in cinnamyl alcohol dehydrogenase, the last enzyme in the monolignol biosynthesis pathway that reduces hydroxycinnamaldehydes to monolignols, chain-incorporated aldehyde unit levels are elevated. The nature and relative levels of aldehyde components in lignins can be determined from their distinct and dispersed correlations in 2D 1H-13C-correlated nuclear magnetic resonance (NMR) spectra. We recently became aware of aldehyde NMR peaks, well resolved from others, that had been overlooked. NMR of isolated low-molecular-weight oligomers from biomimetic radical coupling reactions involving coniferaldehyde revealed that the correlation peaks belonged to hydroxycinnamaldehyde-derived benzofuran moieties. Coniferaldehyde 8-5-coupling initially produces the expected phenylcoumaran structures, but the derived phenolic radicals undergo preferential disproportionation rather than radical coupling to extend the growing polymer. As a result, the hydroxycinnamaldehyde-derived phenylcoumaran units are difficult to detect in lignins, but the benzofurans are now readily observed by their distinct and dispersed correlations in the aldehyde region of NMR spectra from any lignin or monolignol dehydrogenation polymer. Hydroxycinnamaldehydes that are coupled to coniferaldehyde can be distinguished from those coupled with a generic guaiacyl end-unit. These benzofuran peaks may now be annotated and reported and their structural ramifications further studied.
Metabolome and Transcriptome Analysis of Sulfur-Induced Kiwifruit Stem Laccase Gene Involved in Syringyl Lignin Synthesis against Bacterial Canker.[Pubmed:37651104]
J Agric Food Chem. 2023 Sep 13;71(36):13566-13576.
Kiwifruit canker is caused by Pseudomonas syringae pv. actinidiae and is one of the most destructive diseases of kiwifruit worldwide. Sulfur can improve the deposit of lignin in kiwifruit stems and induce disease resistance, but the action mechanism at the molecular level remains unclear. This omics-based study revealed that sulfur-induced S lignin synthesis contributes to disease resistance. Histological staining verified sulfur-enhanced total lignin deposition in kiwifruit stems. High-performance liquid chromatography and confocal Raman microscopy showed that sulfur-activated S lignin was mainly deposited in the cell corner. Metabolome and transcriptome analysis revealed that the levels of phenylpropanoid pathway S lignin precursors sinapic acid and Sinapyl alcohol were significantly increased and 16 laccase genes were upregulated. Sulfur-induced resistance defense promoted elevated laccase activity by activating the laccase genes, participating in sinapic acid and Sinapyl alcohol substance synthesis, and ultimately polymerizing S lignin at cell corner against kiwifruit canker disease.
Integrated metabolomics, transcriptomics, and proteomics analyses reveal co-exposure effects of polycyclic aromatic hydrocarbons and cadmium on ryegrass (Lolium perenne L.).[Pubmed:37517176]
Environ Int. 2023 Aug;178:108105.
Cadmium (Cd) and polycyclic aromatic hydrocarbons (PAHs) are prominent soil contaminants found in industrial sites, and their combined effects on plants are not yet fully understood. To investigate the mechanisms underlying the co-exposure of Cd and PAHs and identify key biomarkers for their co-effects, an integrated analysis of metabolomics, transcriptomics, and proteomics was conducted on ryegrass leaves cultivated in soil. In nontarget metabolomics analysis, nine differentially expressed metabolites that were specifically induced by the compound exposure were identified. When combined with the analysis of differentially expressed genes and proteins, it was determined that the major pathways involved in the response to the co-stress of Cd and PAHs were linoleic acid metabolism and phenylpropanoid biosynthesis. The upregulation of 12,13-dihydroxy-9Z-octadecenoic acid and the downregulation of Sinapyl alcohol were identified as typical biomarkers, respectively. Compared to scenarios of single exposures, the compound exposure to Cd and PAHs disrupted the oxidation of linoleic acid, leading to alterations in the profiles of linoleate metabolites. Additionally, it intensified hydroxylation, carboxylation, and methylation processes, and interfered with reactions involving coenzyme A, thus inhibiting lignin production. As a result, oxidative stress was elevated, and the cell wall defense system in ryegrass was weakened. The findings of this study highlight the ecological risks associated with unique biological responses in plants co-exposed to Cd and PAHs in polluted soils.
Pinoresinol rescues developmental phenotypes of Arabidopsis phenylpropanoid mutants overexpressing FERULATE 5-HYDROXYLASE.[Pubmed:37487096]
Proc Natl Acad Sci U S A. 2023 Aug;120(31):e2216543120.
Most phenylpropanoid pathway flux is directed toward the production of monolignols, but this pathway also generates multiple bioactive metabolites. The monolignols coniferyl and Sinapyl alcohol polymerize to form guaiacyl (G) and syringyl (S) units in lignin, components that are characteristic of plant secondary cell walls. Lignin negatively impacts the saccharification potential of lignocellulosic biomass. Although manipulation of its content and composition through genetic engineering has reduced biomass recalcitrance, in some cases, these genetic manipulations lead to impaired growth. The reduced-growth phenotype is often attributed to poor water transport due to xylem collapse in low-lignin mutants, but alternative models suggest that it could be caused by the hyper- or hypoaccumulation of phenylpropanoid intermediates. In Arabidopsis thaliana, overexpression of FERULATE 5-HYDROXYLASE (F5H) shifts the normal G/S lignin ratio to nearly pure S lignin and does not result in substantial changes to plant growth. In contrast, when we overexpressed F5H in the low-lignin mutants cinnamyl dehydrogenase c and d (cadc cadd), cinnamoyl-CoA reductase 1, and reduced epidermal fluorescence 3, plant growth was severely compromised. In addition, cadc cadd plants overexpressing F5H exhibited defects in lateral root development. Exogenous coniferyl alcohol (CA) and its dimeric coupling product, pinoresinol, rescue these phenotypes. These data suggest that mutations in the phenylpropanoid pathway limit the biosynthesis of pinoresinol, and this effect is exacerbated by overexpression of F5H, which further draws down cellular pools of its precursor, CA. Overall, these genetic manipulations appear to restrict the synthesis of pinoresinol or a downstream metabolite that is necessary for plant growth.
Transcriptomic and metabolomic analyses reveal the potential mechanism of waterlogging resistance in cotton (Gossypium hirsutum L.).[Pubmed:37409297]
Front Plant Sci. 2023 Jun 12;14:1088537.
INTRODUCTION: Cotton (Gossypium hirsutum L.) is susceptible to long-term waterlogging stress; however, genomic information of cotton response mechanisms toward long days of waterlogging is quite elusive. METHODS: Here, we combined the transcriptome and metabolome expression level changes in cotton roots after 10 and 20 days of waterlogging stress treatment pertaining to potential resistance mechanisms in two cotton genotypes. RESULTS AND DISCUSSION: Numerous adventitious roots and hypertrophic lenticels were induced in CJ1831056 and CJ1831072. Transcriptome analysis revealed 101,599 differentially expressed genes in cotton roots with higher gene expression after 20 days of stress. Reactive oxygen species (ROS) generating genes, antioxidant enzyme genes, and transcription factor genes (AP2, MYB, WRKY, and bZIP) were highly responsive to waterlogging stress among the two genotypes. Metabolomics results showed higher expressions of stress-resistant metabolites Sinapyl alcohol, L-glutamic acid, galactaric acid, glucose 1-phosphate, L-valine, L-asparagine, and melibiose in CJ1831056 than CJ1831072. Differentially expressed metabolites (adenosine, galactaric acid, Sinapyl alcohol, L-valine, L-asparagine, and melibiose) significantly correlated with the differentially expressed PRX52, PER1, PER64, and BGLU11 transcripts. This investigation reveals genes for targeted genetic engineering to improve waterlogging stress resistance to enhance abiotic stress regulatory mechanisms in cotton at the transcript and metabolic levels of study.
Integrated non-targeted and targeted metabolomics analysis reveals the mechanism of inhibiting lignification and optimizing the quality of pea sprouts by combined application of nano-selenium and lentinans.[Pubmed:36974656]
J Sci Food Agric. 2023 Aug 15;103(10):5096-5107.
BACKGROUND: Lignification causes a detrimental impact on the quality of edible sprouts. However, the mechanism of inhibition of lignification of edible sprouts by nano-selenium and lentinans remains unclear. RESULTS: To reveal the mechanism of lignification regulation of sprouts by nano-selenium and lentinans, this study investigated the changes in antioxidant indicators, phytohormones, polyphenols, and metabolites in the lignin biosynthesis in pea sprouts following sprays of nano-selenium or/and lentinans twice. There was an overall increase in the aforementioned indices following treatment. In particular, the combined application of 5 mg L(-1) nano-selenium and 20 mg L(-1) lentinans was more effective than their individual applications in enhancing peroxidase, catalase, DPPH free-radical scavenging rate, luteolin, and sinapic acid, as well as inhibiting malondialdehyde generation and lignin accumulation. Combined with the results from correlation analysis, nano-selenium and lentinans may inhibit lignification by enhancing antioxidant systems, inducing phytohormone-mediated signaling, and enriching precursor metabolites (caffeyl alcohol, Sinapyl alcohol, 4-coumaryl alcohol). In terms of the results of non-targeted metabolomics, the combined application of 5 mg L(-1) nano-selenium and 20 mg L(-1) lentinans mainly affected biosynthesis of plant secondary metabolites, biosynthesis of phenylpropanoids, phenylpropanoid biosynthesis, arginine and proline metabolism, and linoleic acid metabolism pathways, which supported and complemented results from targeted screenings. CONCLUSION: Overall, the combined sprays of nano-selenium and lentinans showed synergistic effects in delaying lignification and optimizing the quality of pea sprouts. This study provides a novel and practicable technology for delaying lignification in the cultivation of edible sprouts. (c) 2023 Society of Chemical Industry.
Biomimetic oxidative copolymerization of hydroxystilbenes and monolignols.[Pubmed:36888720]
Sci Adv. 2023 Mar 10;9(10):eade5519.
Hydroxystilbenes are a class of polyphenolic compounds that behave as lignin monomers participating in radical coupling reactions during the lignification. Here, we report the synthesis and characterization of various artificial copolymers of monolignols and hydroxystilbenes, as well as low-molecular-mass compounds, to obtain the mechanistic insights into their incorporation into the lignin polymer. Integrating the hydroxystilbenes, resveratrol and piceatannol, into monolignol polymerization in vitro, using horseradish peroxidase to generate phenolic radicals, produced synthetic lignins [dehydrogenation polymers (DHPs)]. Copolymerization of hydroxystilbenes with monolignols, especially Sinapyl alcohol, by in vitro peroxidases notably improved the reactivity of monolignols and resulted in substantial yields of synthetic lignin polymers. The resulting DHPs were analyzed using two-dimensional NMR and 19 synthesized model compounds to confirm the presence of hydroxystilbene structures in the lignin polymer. The cross-coupled DHPs confirmed both resveratrol and piceatannol as authentic monomers participating in the oxidative radical coupling reactions during polymerization.


