SimiarenolCAS# 1615-94-7 |
Quality Control & MSDS
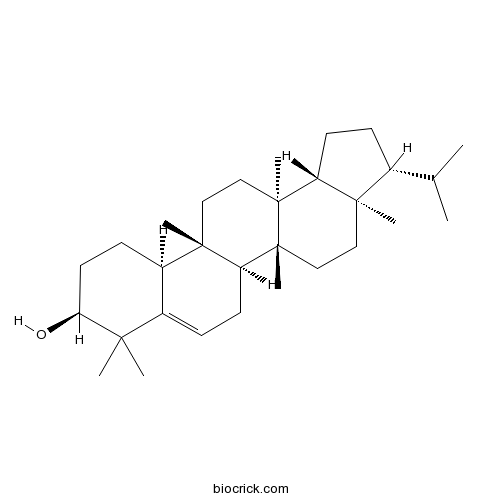
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1615-94-7 | SDF | Download SDF |
| PubChem ID | 12442794 | Appearance | Cryst. |
| Formula | C30H50O | M.Wt | 426.7 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3R,3aR,5aR,5bS,9S,11aS,11bR,13aS,13bR)-3a,5a,8,8,11b,13a-hexamethyl-3-propan-2-yl-1,2,3,4,5,5b,6,9,10,11,11a,12,13,13b-tetradecahydrocyclopenta[a]chrysen-9-ol | ||
| SMILES | CC(C)C1CCC2C1(CCC3(C2(CCC4(C3CC=C5C4CCC(C5(C)C)O)C)C)C)C | ||
| Standard InChIKey | XVXPXUMUGATHPD-JMJRLLIOSA-N | ||
| Standard InChI | InChI=1S/C30H50O/c1-19(2)20-9-12-23-27(20,5)15-17-30(8)24-13-10-21-22(11-14-25(31)26(21,3)4)28(24,6)16-18-29(23,30)7/h10,19-20,22-25,31H,9,11-18H2,1-8H3/t20-,22-,23-,24+,25+,27-,28+,29+,30-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Simiarenol may have in vitro leishmanicidal activity against Leishmania donovani promastigotes. |
| Targets | Antifection |

Simiarenol Dilution Calculator

Simiarenol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3436 mL | 11.7178 mL | 23.4357 mL | 46.8713 mL | 58.5892 mL |
| 5 mM | 0.4687 mL | 2.3436 mL | 4.6871 mL | 9.3743 mL | 11.7178 mL |
| 10 mM | 0.2344 mL | 1.1718 mL | 2.3436 mL | 4.6871 mL | 5.8589 mL |
| 50 mM | 0.0469 mL | 0.2344 mL | 0.4687 mL | 0.9374 mL | 1.1718 mL |
| 100 mM | 0.0234 mL | 0.1172 mL | 0.2344 mL | 0.4687 mL | 0.5859 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4-Chlorocinnamic acid
Catalog No.:BCN5032
CAS No.:1615-02-7
- 7-NINA
Catalog No.:BCC5674
CAS No.:161467-34-1
- SIN-1 chloride
Catalog No.:BCC5670
CAS No.:16142-27-1
- ABT
Catalog No.:BCC7998
CAS No.:1614-12-6
- HTH-01-015
Catalog No.:BCC4010
CAS No.:1613724-42-7
- SGC-CBP30
Catalog No.:BCC2419
CAS No.:1613695-14-9
- Fmoc-Chg-OH
Catalog No.:BCC3164
CAS No.:161321-36-4
- 1-O-Acetyl-6-O-isobutyrylbritannilactone
Catalog No.:BCN7795
CAS No.:1613152-34-3
- SR-9243
Catalog No.:BCC3983
CAS No.:1613028-81-1
- H-Orn(Z)-OtBu.HCl
Catalog No.:BCC2677
CAS No.:161234-80-6
- Cimicidanol 3-O-alpha-L-arabinoside
Catalog No.:BCN6528
CAS No.:161207-05-2
- H-Asp(OEt)-OEt.HCl
Catalog No.:BCC2888
CAS No.:16115-68-7
- Fmoc-Alaninol
Catalog No.:BCC2729
CAS No.:161529-13-1
- Cyclo (-RGDfK)
Catalog No.:BCC3590
CAS No.:161552-03-0
- Ro 48-8071
Catalog No.:BCC5545
CAS No.:161582-11-2
- (S)-N-Glycidylphthalimide
Catalog No.:BCN3815
CAS No.:161596-47-0
- 2'',3''-Di-O-acetyl-5''-deoxy-5-fuluro-D-cytidine
Catalog No.:BCN1545
CAS No.:161599-46-8
- beta-Amyrin acetate
Catalog No.:BCN1715
CAS No.:1616-93-9
- ZK 200775
Catalog No.:BCC7339
CAS No.:161605-73-8
- Epimedonin B
Catalog No.:BCN7889
CAS No.:1616061-69-8
- Hispidanin B
Catalog No.:BCN7394
CAS No.:1616080-84-2
- VRT-1353385
Catalog No.:BCC6433
CAS No.:1616113-45-1
- EPZ015666
Catalog No.:BCC5653
CAS No.:1616391-65-1
- Erythrinin D
Catalog No.:BCN6858
CAS No.:1616592-59-6
Antiproliferative constituents of the roots of Conyza canadensis.[Pubmed:21294076]
Planta Med. 2011 Jul;77(11):1183-8.
Bioassay-guided fractionation of the N-hexane and CHCl(3) phases of the methanol extract of the roots of Conyza canadensis (L.) Cronquist led to the isolation of two new dihydropyranones named conyzapyranone A (1) and B (2), and the known 4 Z,8 Z-matricaria- gamma-lactone (3), 4 E,8 Z-matricaria- gamma-lactone (4), 9,12,13-trihydroxy-10(E)-octadecenoic acid (5), epifriedelanol (6), friedeline (7), taraxerol (8), Simiarenol (9), spinasterol (10), stigmasterol, beta-sitosterol, and apigenin. The structures were determined by means of ESIMS and 1D and 2D NMR spectroscopy, including (1)H-(1)H COSY, NOESY, HSQC, and HMBC experiments. The isolated compounds were evaluated for their antiproliferative activities and were demonstrated to exert considerable cell growth-inhibitory activity against human cervix adenocarcinoma (HeLa), skin carcinoma (A431), and breast adenocarcinoma (MCF-7) cells. Some of the active components, including 2, 4, and 10, proved to be substantially more potent against these cell lines than against noncancerous human foetal fibroblasts (MRC-5) and can therefore be considered selective antiproliferative natural products.
3 beta-hydroxy-28-P-coumaroyloxy-lup-20(29)-en-27-oic acid from Caraipa densifolia.[Pubmed:6854336]
J Nat Prod. 1983 Jan;46(1):118-22.
Caraipa densifolia Mart. (Clusiaceae) has afforded the new lupene derivative 3 beta-hydroxy-28-p-coumarolyoxy-lup-20(29)-en-27-oic acid (8), whose structure was deduced by chemical correlation with betulin (6). Simiarenol (1), taraxerone (2), friedelin (3), lupeol (4), betulinic acid (5), betulin (6), and beta-sitosterol-beta-D-glucoside (7) were also isolated in this study.
[Chemical constituents from Imperata cylindrica].[Pubmed:23189737]
Zhongguo Zhong Yao Za Zhi. 2012 Aug;37(15):2296-300.
Chemical investigation of Imperata cylindrica led to the isolation of thirteen compounds using various chromatographic techniques. The structure of these compounds were identified as: three phenylpropanoids, 1-(3,4,5-trimethoxyphenyl)-1,2,3-propanetriol ( 1 ), 1-O-p-coumaroylglycerol (2), 4-methoxy-5-methyl coumarin-7-O-beta-D-glucopyranoside (3); four organic acids, 4-hydroxybenzene carboxylic acid(4), 3,4-dihydroxybenzoic acid (5), vanillic acid (6), 3, 4-dihydroxybutyric acid (7); one phenolic compound, salicin (8); and five triterpenes, namely, arundoin (9), cylindrin (10), fernenol (11), Simiarenol (12), glutinone (13) by their physicochemical properties and spectral data analysis. Among them, compounds 1-8 were isolated from the genus Imperata for the first time.
Biologically-guided isolation of leishmanicidal secondary metabolites from Euphorbia peplus L.[Pubmed:28344474]
Saudi Pharm J. 2017 Feb;25(2):236-240.
Leishmaniasis is a worldwide health problem, highly endemic in developing countries. Moreover, the severe side effects and the reported drug resistance make it an urgent need to search for effective drugs that can replace or supplement those currently used. In a research program designed to investigate the antileishmanial activity of plants collected from the Egyptian flora, twenty extracts from fifteen plants growing in Egypt have been investigated for in vitro leishmanicidal activity against Leishmania donovani promastigotes. Among the tested extracts, the methanol extract of Euphorbia peplus aerial parts exhibited a significant antileishmanial activity as it produced 100% inhibition of growth with activity similar to amphotericin B. The total extract was subjected to liquid-liquid fractionation using solvents of different polarities, followed by testing the antileishmanial activity of the successive fractions. Phytochemical exploration of the active n-hexane fraction (which produced 75% inhibition of growth) led to isolation of four compounds: Simiarenol (1), 1-hexacosanol (2), beta-sitosterol (3), and beta-sitosterol-3-O-glucoside (4) from the biologically active sub-fractions. Structure elucidation was aided by 1D and 2D NMR techniques. In conclusion, E. peplus plant has many non-polar secondary metabolites that can be used as drug leads for treatment of leishmaniasis.
Phytochemical constituents of Artemisia stolonifera.[Pubmed:11534763]
Arch Pharm Res. 2001 Aug;24(4):312-5.
Repeated column chromatographic separation of the CH2Cl2 extract of Artemisia stolonifera (Asteraceae) led to the isolation of a triterpene (I), a sesquiterpene (II), two aromatic compounds (III and IV) and a benzoquinone (V). Their structures were determined by spectroscopic means to be Simiarenol (I), (1S,7S)-1beta-hydroxygermacra-4(15),5,10(14)-triene (II), 3'-methoxy-4'-hydroxy-trans-cinnamaldehyde (III), vanillin (IV) and 2,6-dimethoxy-1,4-benzoquinone (V), respectively. Among these products, compound V showed significant cytotoxicity against five human tumor cell lines in vitro, A549 (non small cell lung adenocarcinoma), SK-OV-3 (ovarian), SK-MEL-2 (skin melanoma), XF498 (CNS) and HCT15 (colon) with ED50 values ranging from 1.33-4.22 microg/ml.


