SR 12813Enhances HMG-CoA reductase degradation. Also PXR agonist CAS# 126411-39-0 |
- PA-824
Catalog No.:BCC1106
CAS No.:187235-37-6
- Clofazimine
Catalog No.:BCC4651
CAS No.:2030-63-9
- 5-hydroxypyrazine-2-carboxylic acid
Catalog No.:BCC1311
CAS No.:34604-60-9
- Nitazoxanide
Catalog No.:BCC3824
CAS No.:55981-09-4
- Sodium 4-Aminosalicylate
Catalog No.:BCC4609
CAS No.:6018-19-5
- Rifapentine
Catalog No.:BCC4937
CAS No.:61379-65-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 126411-39-0 | SDF | Download SDF |
| PubChem ID | 446313 | Appearance | Powder |
| Formula | C24H42O7P2 | M.Wt | 504.53 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | GW 485801 | ||
| Solubility | DMSO : ≥ 50 mg/mL (99.10 mM) *"≥" means soluble, but saturation unknown. | ||
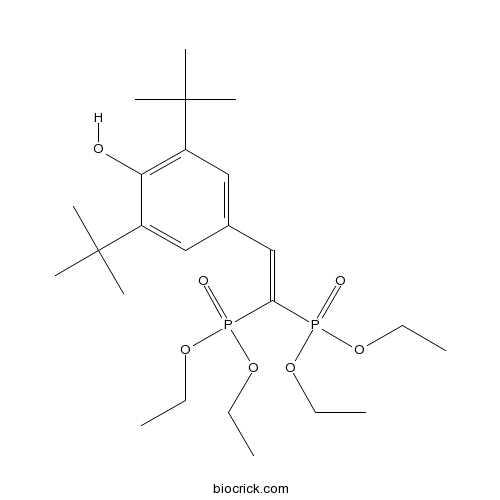
| Chemical Name | 4-[2,2-bis(diethoxyphosphoryl)ethenyl]-2,6-ditert-butylphenol | ||
| SMILES | CCOP(=O)(C(=CC1=CC(=C(C(=C1)C(C)(C)C)O)C(C)(C)C)P(=O)(OCC)OCC)OCC | ||
| Standard InChIKey | YQLJDECYQDRSBI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C24H42O7P2/c1-11-28-32(26,29-12-2)21(33(27,30-13-3)31-14-4)17-18-15-19(23(5,6)7)22(25)20(16-18)24(8,9)10/h15-17,25H,11-14H2,1-10H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Pregnane X receptor (PXR) agonist (EC50 values are 200 and 700 nM for human and rabbit PXR respectively). Activates the farnesoid X receptor (FXR) at μM concentrations. Displays hypocholesterolaemic activity via enchanced degradation of HMG-CoA reductase. |

SR 12813 Dilution Calculator

SR 12813 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.982 mL | 9.9102 mL | 19.8204 mL | 39.6409 mL | 49.5511 mL |
| 5 mM | 0.3964 mL | 1.982 mL | 3.9641 mL | 7.9282 mL | 9.9102 mL |
| 10 mM | 0.1982 mL | 0.991 mL | 1.982 mL | 3.9641 mL | 4.9551 mL |
| 50 mM | 0.0396 mL | 0.1982 mL | 0.3964 mL | 0.7928 mL | 0.991 mL |
| 100 mM | 0.0198 mL | 0.0991 mL | 0.1982 mL | 0.3964 mL | 0.4955 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
SR12813 is an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, with an IC50 value of 0.85 μM.
In Vitro:SR-12813 inhibits incorporation of tritiated water into cholesterol with an IC50 of 1.2 μM but has no effect on fatty acid synthesis. Furthermore, SR-12813 reduces cellular 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase activity with an IC50 of 0.85 μM[1]. Both 25-HC and SR-12813 can kill mammalian cells through blocking the synthesis of cholesterol, thereby they are ideal regents for lethal selection. SR-12813 kills HeLa cells at concentration range from 8 µM to 16 µM. SR-12813 kills wild type cells and mutant cells infected by Ad-Cre (SL-5+Cre), but the mutant SL-5 survives this condition. SR-12813 or 25-HC promotes the degradation of the 95-KDa full-length HMG-CoA reductase in wild type HeLa and SL-5 mutant cells[1].
References:
[1]. Berkhout T, et al. The novel cholesterol-lowering drug SR-12813 inhibits cholesterol synthesis via an increased degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J Biol Chem. 1996 Jun 14;271(24):14376-82.
[2]. Jiang W, et al. Forward genetic screening for regulators involved in cholesterol synthesis using validation-based insertional mutagenesis. PLoS One. 2014 Nov 26;9(11):e112632.
- Colistin Sulfate
Catalog No.:BCC4653
CAS No.:1264-72-8
- Nortropinyl cinnamate
Catalog No.:BCN1891
CAS No.:126394-79-4
- Pinobanksin 3-O-propanoate
Catalog No.:BCN7737
CAS No.:126394-70-5
- CDK9 inhibitor 2
Catalog No.:BCC1466
CAS No.:1263369-28-3
- 7-Chlorokynurenic acid sodium salt
Catalog No.:BCC7757
CAS No.:1263094-00-3
- DL-AP4 Sodium salt
Catalog No.:BCC7759
CAS No.:1263093-79-3
- Paromomycin Sulfate
Catalog No.:BCC4694
CAS No.:1263-89-4
- NCX 466
Catalog No.:BCC6219
CAS No.:1262956-64-8
- Spautin-1
Catalog No.:BCC6420
CAS No.:1262888-28-7
- CCT241533
Catalog No.:BCC1462
CAS No.:1262849-73-9
- 25-Hydroxy VD2-D6
Catalog No.:BCC1305
CAS No.:1262843-46-8
- Trigonosin F
Catalog No.:BCN6403
CAS No.:1262842-73-8
- VTP-27999 Hydrochloride
Catalog No.:BCC2050
CAS No.:1264191-73-2
- Pyrrolam A
Catalog No.:BCN2040
CAS No.:126424-76-8
- Pyrrolam B
Catalog No.:BCN1986
CAS No.:126424-77-9
- 6-Ethoxygeniposide
Catalog No.:BCN7043
CAS No.:1264496-61-8
- (R)-CPP
Catalog No.:BCC6581
CAS No.:126453-07-4
- (1S,2R)-1-Amino-2-indanol
Catalog No.:BCC8384
CAS No.:126456-43-7
- Dihydroeponemycin
Catalog No.:BCC3596
CAS No.:126463-64-7
- Metasequirin D
Catalog No.:BCN4781
CAS No.:1264694-96-3
- Mupirocin
Catalog No.:BCC5558
CAS No.:12650-69-0
- CH5183284 (Debio-1347)
Catalog No.:BCC5649
CAS No.:1265229-25-1
- Ciclesonide
Catalog No.:BCC5234
CAS No.:126544-47-6
- 8,14-Epoxyergosta-4,22-diene-3,6-dione
Catalog No.:BCN1591
CAS No.:1265908-20-0
The novel cholesterol-lowering drug SR-12813 inhibits cholesterol synthesis via an increased degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase.[Pubmed:8662919]
J Biol Chem. 1996 Jun 14;271(24):14376-82.
SR-12813 (tetra-ethyl 2-(3,5-di-tert-butyl-4-hydroxyphenyl)ethenyl-1, 1-bisphosphonate) lowers plasma cholesterol in five species. In this paper we investigate the underlying mechanism using Hep G2 cells. SR-12813 inhibited incorporation of tritiated water into cholesterol with an IC50 of 1.2 microM but had no effect on fatty acid synthesis. Furthermore, SR-12813 reduced cellular 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase activity with an IC50 of 0.85 microM. The inhibition of HMG-CoA reductase activity was rapid with a T1/2 of 10 min. After a 16-h incubation with SR-12813, mRNA levels of HMG-CoA reductase and low density lipoprotein (LDL) receptor were increased. The increased expression of LDL receptor translated into a higher LDL uptake, which can explain the primary hypocholesterolemic effect of SR-12813 in vivo. Western blot analysis indicated that the amount of HMG-CoA reductase protein rapidly decreased in the presence of SR-12813. Pulse-chase experiments with [35S]methionine showed that the T1/2 of HMG-CoA reductase degradation decreased in the presence of SR-12813 from 90 to 20 min. Pre-incubation with 50 microM of lovastatin did not prevent the effects of SR-12813 on HMG-CoA reductase degradation, indicating that the compound does not need mevalonate-derived regulators for its action. It is concluded that SR-12813 inhibits cholesterol synthesis mainly by an enhanced degradation of HMG-CoA reductase.
SR-12813 lowers plasma cholesterol in beagle dogs by decreasing cholesterol biosynthesis.[Pubmed:9298680]
Atherosclerosis. 1997 Sep;133(2):203-12.
SR-12813 inhibits cholesterol biosynthesis in Hep G2 cells via an enhanced degradation of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase. Here we also show that SR-12813 inhibits cholesterol biosynthesis in vivo. A sterol balance study was performed in normolipemic beagle dogs. The dogs were given SR-12813 orally at dosages of 10 and 25 mg/kg/day for a period of 9 days. After 7 days plasma cholesterol was decreased by 15% in the 10 mg/kg/day group and by 19% in the 25 mg/kg/day group. Using a dual isotope technique no effects on intestinal cholesterol absorption were observed. The sterol balance indicated that endogenous synthesis of cholesterol was reduced by 23% in the 10 mg/kg/day group and by 37% in the 25 mg/kg/day group. Plasma lathosterol-cholesterol levels in dogs treated with 25 mg/kg/day SR-12813 were reduced by 56%, confirming a reduction of the cholesterol biosynthesis. Treatment with SR-12813 or the HMG-CoA reductase inhibitor lovastatin resulted in a large decrease in low density lipoprotein (LDL) cholesterol. It is concluded that SR-12813 reduces cholesterol biosynthesis in the dog model which results in a decrease of bile acid excretion, cholesterol excretion and plasma cholesterol level. The in vivo profile of SR-12813 is very similar to that of direct HMG-CoA reductase inhibitors, although the mode of action of the compound is unique.
Isolation of mutant cells lacking Insig-1 through selection with SR-12813, an agent that stimulates degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase.[Pubmed:15247248]
J Biol Chem. 2004 Oct 8;279(41):43136-47.
Insig-1 and Insig-2 are membrane proteins of the endoplasmic reticulum that regulate lipid metabolism by the following two actions: 1) sterol-induced binding to 3-hydroxy-3-methylglutaryl-coenzyme A reductase, an action that leads to ubiquitination and degradation of the enzyme; and 2) sterol-induced binding to SREBP cleavage-activating protein, an action that blocks the proteolytic processing of sterol regulatory element-binding proteins (SREBPs), membrane-bound transcription factors that enhance the synthesis of cholesterol and fatty acids. Here we report the isolation of a new mutant line of Chinese hamster ovary cells, designated SRD-14, in which Insig-1 mRNA and protein are not produced due to a partial deletion of the INSIG-1 gene. The SRD-14 cells were produced by gamma-irradiation, followed by selection with the 1,1-bisphosphonate ester SR-12813, which mimics sterols in accelerating reductase degradation but does not block SREBP processing. SRD-14 cells fail to respond to sterols by promoting reductase ubiquitination and degradation. The rate at which sterols suppress SREBP processing is significantly slower in SRD-14 cells than wild type CHO-7 cells. Sterol regulation of reductase degradation and SREBP processing is restored when SRD-14 cells are transfected with expression plasmids encoding either Insig-1 or Insig-2. These results provide formal genetic proof for the essential role of Insig-1 in feedback control of lipid synthesis in cultured cells.
Discovery of a highly active ligand of human pregnane x receptor: a case study from pharmacophore modeling and virtual screening to "in vivo" biological activity.[Pubmed:17573484]
Mol Pharmacol. 2007 Sep;72(3):572-81.
The human pregnane X receptor (hPXR) is a nuclear receptor that regulates the expression of phase I and II drug-metabolizing enzymes as well as that of drug transporters. In addition, this receptor plays a critical role in cholesterol homeostasis and in protecting tissues from potentially toxic endobiotics. hPXR is activated by a broad spectrum of low-affinity compounds including xenobiotics and endobiotics such as bile acids and their precursors. Crystallographic studies revealed a ligand binding domain (LBD) with a large and conformable binding pocket that is likely to contribute to the ability of hPXR to respond to compounds of varying size and shape. Here, we describe an in silico method that allowed the identification of nine novel hPXR agonists. We further characterize the compound 1-(2-chlorophenyl)-N-[1-(1-phenylethyl)-1H-benzimidazol-5-yl]methanesulfonamide (C2BA-4), a methanesulfonamide that activates PXR specifically and more potently than does the reference compound 4-[2,2-bis(diethoxyphosphoryl)ethenyl]-2,6-ditert-butyl-phenol (SR12813) in our stable cell line expressing a Gal4-PXR and a GAL4 driven luciferase reporter gene. Furthermore treatment of primary human hepatocytes with C2BA-4 results in a marked induction of the mRNA expression of hPXR target genes, such as cytochromes P450 3A4 and 2B6. Finally, C2BA-4 is also able to induce hPXR-mediated in vivo luciferase expression in HGPXR stable bioluminescent cells implanted in mice. The study suggests new directions for the rational design of selective hPXR agonists and antagonists.


