Pyrrolam ACAS# 126424-76-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 126424-76-8 | SDF | Download SDF |
| PubChem ID | 5325756 | Appearance | Cryst. |
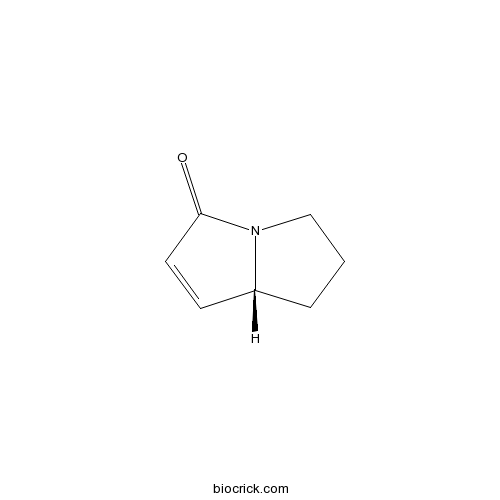
| Formula | C7H9NO | M.Wt | 123.15 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (8R)-5,6,7,8-tetrahydropyrrolizin-3-one | ||
| SMILES | C1CC2C=CC(=O)N2C1 | ||
| Standard InChIKey | FVZBHZCORGROSI-ZCFIWIBFSA-N | ||
| Standard InChI | InChI=1S/C7H9NO/c9-7-4-3-6-2-1-5-8(6)7/h3-4,6H,1-2,5H2/t6-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| In vitro | Synthesis of (R)‐(‐)‐Pyrrolam A (I) and Studies on Its Stability: A Caveat on Computational Methods[Reference: WebLink]The Journal of Organic Chemistry,2004, 69(18):6105-14.The asymmetric synthesis of (-)-(R)-Pyrrolam A was achieved in three operations from N-Boc pyrrolidine via an alpha-(N-carbamoyl)alkylcuprate vinylation reaction followed by N-Boc deprotection and cyclization. One-pot deprotection-cyclization procedures led to mixtures of Pyrrolam A and its double bond isomers. These isomerization events could be circumvented by use of a two-step procedure. |
| Structure Identification | Org Lett. 2014 Jul 18;16(14):3780-3.Enantioselective catalytic desymmetrization of maleimides by temporary removal of an internal mirror plane and stereoablative over-reduction: synthesis of (R)-pyrrolam A.[Pubmed: 24972082]
Tetrahedron,1996,52(3): 869–876.First total synthesis of pyrrolam A.[Reference: WebLink]
|

Pyrrolam A Dilution Calculator

Pyrrolam A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 8.1202 mL | 40.6009 mL | 81.2018 mL | 162.4036 mL | 203.0045 mL |
| 5 mM | 1.624 mL | 8.1202 mL | 16.2404 mL | 32.4807 mL | 40.6009 mL |
| 10 mM | 0.812 mL | 4.0601 mL | 8.1202 mL | 16.2404 mL | 20.3004 mL |
| 50 mM | 0.1624 mL | 0.812 mL | 1.624 mL | 3.2481 mL | 4.0601 mL |
| 100 mM | 0.0812 mL | 0.406 mL | 0.812 mL | 1.624 mL | 2.03 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- VTP-27999 Hydrochloride
Catalog No.:BCC2050
CAS No.:1264191-73-2
- SR 12813
Catalog No.:BCC7530
CAS No.:126411-39-0
- Colistin Sulfate
Catalog No.:BCC4653
CAS No.:1264-72-8
- Nortropinyl cinnamate
Catalog No.:BCN1891
CAS No.:126394-79-4
- Pinobanksin 3-O-propanoate
Catalog No.:BCN7737
CAS No.:126394-70-5
- CDK9 inhibitor 2
Catalog No.:BCC1466
CAS No.:1263369-28-3
- 7-Chlorokynurenic acid sodium salt
Catalog No.:BCC7757
CAS No.:1263094-00-3
- DL-AP4 Sodium salt
Catalog No.:BCC7759
CAS No.:1263093-79-3
- Paromomycin Sulfate
Catalog No.:BCC4694
CAS No.:1263-89-4
- NCX 466
Catalog No.:BCC6219
CAS No.:1262956-64-8
- Spautin-1
Catalog No.:BCC6420
CAS No.:1262888-28-7
- CCT241533
Catalog No.:BCC1462
CAS No.:1262849-73-9
- Pyrrolam B
Catalog No.:BCN1986
CAS No.:126424-77-9
- 6-Ethoxygeniposide
Catalog No.:BCN7043
CAS No.:1264496-61-8
- (R)-CPP
Catalog No.:BCC6581
CAS No.:126453-07-4
- (1S,2R)-1-Amino-2-indanol
Catalog No.:BCC8384
CAS No.:126456-43-7
- Dihydroeponemycin
Catalog No.:BCC3596
CAS No.:126463-64-7
- Metasequirin D
Catalog No.:BCN4781
CAS No.:1264694-96-3
- Mupirocin
Catalog No.:BCC5558
CAS No.:12650-69-0
- CH5183284 (Debio-1347)
Catalog No.:BCC5649
CAS No.:1265229-25-1
- Ciclesonide
Catalog No.:BCC5234
CAS No.:126544-47-6
- 8,14-Epoxyergosta-4,22-diene-3,6-dione
Catalog No.:BCN1591
CAS No.:1265908-20-0
- S1RA hydrochloride
Catalog No.:BCC4190
CAS No.:1265917-14-3
- Cerberidol
Catalog No.:BCN6142
CAS No.:126594-64-7
Enantioselective catalytic desymmetrization of maleimides by temporary removal of an internal mirror plane and stereoablative over-reduction: synthesis of (R)-pyrrolam A.[Pubmed:24972082]
Org Lett. 2014 Jul 18;16(14):3780-3.
A highly enantioselective (>95% ee) strategy to affect the desymmetrization of a maleimide has been performed by temporary attachment to an anthracene template followed by asymmetric reduction with an oxazaborolidine catalyst. A stereoablative over-reduction process was partially responsible for the high levels of enantioselectivity. Exemplification of the strategy by stereoselective functionalization and retro-Diels-Alder reaction provided the natural product Pyrrolam A.


