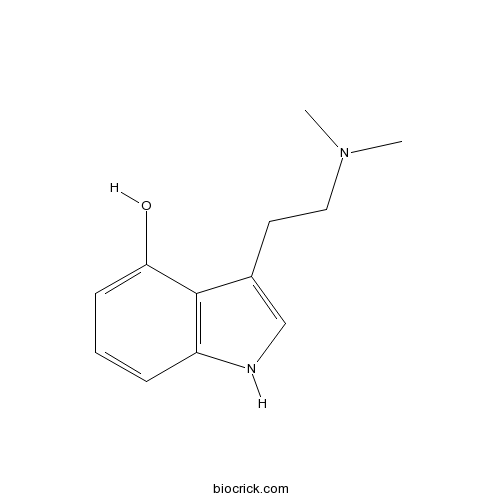
PsilocinNon-selective serotonin receptor agonist CAS# 520-53-6 |
- MLN9708
Catalog No.:BCC2091
CAS No.:1201902-80-8
- MG-132
Catalog No.:BCC1227
CAS No.:133407-82-6
- Clasto-Lactacystin β-lactone
Catalog No.:BCC1224
CAS No.:154226-60-5
- Bortezomib (PS-341)
Catalog No.:BCC1238
CAS No.:179324-69-7
- CEP-18770
Catalog No.:BCC2093
CAS No.:847499-27-8
- AM 114
Catalog No.:BCC3589
CAS No.:856849-35-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 520-53-6 | SDF | Download SDF |
| PubChem ID | 4980 | Appearance | Powder |
| Formula | C12H16N2O | M.Wt | 204.27 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
| Chemical Name | 3-[2-(dimethylamino)ethyl]-1H-indol-4-ol | ||
| SMILES | CN(C)CCC1=CNC2=C1C(=CC=C2)O | ||
| Standard InChIKey | SPCIYGNTAMCTRO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H16N2O/c1-14(2)7-6-9-8-13-10-4-3-5-11(15)12(9)10/h3-5,8,13,15H,6-7H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Non-selective serotonin receptor agonist. Active metabolite of psilocybin; hallucinogen. |

Psilocin Dilution Calculator

Psilocin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.8955 mL | 24.4774 mL | 48.9548 mL | 97.9096 mL | 122.387 mL |
| 5 mM | 0.9791 mL | 4.8955 mL | 9.791 mL | 19.5819 mL | 24.4774 mL |
| 10 mM | 0.4895 mL | 2.4477 mL | 4.8955 mL | 9.791 mL | 12.2387 mL |
| 50 mM | 0.0979 mL | 0.4895 mL | 0.9791 mL | 1.9582 mL | 2.4477 mL |
| 100 mM | 0.049 mL | 0.2448 mL | 0.4895 mL | 0.9791 mL | 1.2239 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Asebogenin
Catalog No.:BCN7232
CAS No.:520-42-3
- Apigenin
Catalog No.:BCN5658
CAS No.:520-36-5
- Diosmetin
Catalog No.:BCN2356
CAS No.:520-34-3
- Hesperetin
Catalog No.:BCN5657
CAS No.:520-33-2
- Tricin
Catalog No.:BCN5656
CAS No.:520-32-1
- Tectochrysin
Catalog No.:BCN5655
CAS No.:520-28-5
- Diosimin
Catalog No.:BCN4993
CAS No.:520-27-4
- Hesperidin
Catalog No.:BCN5654
CAS No.:520-26-3
- Kaempferol
Catalog No.:BCN5653
CAS No.:520-18-3
- Pectolinarigenin
Catalog No.:BCN5813
CAS No.:520-12-7
- 6-Methoxyluteolin
Catalog No.:BCN3613
CAS No.:520-11-6
- H-Cys-OH
Catalog No.:BCC2902
CAS No.:52-90-4
- Spectabiline
Catalog No.:BCN2098
CAS No.:520-55-8
- Spartioidine
Catalog No.:BCN2134
CAS No.:520-59-2
- Rosmarinine
Catalog No.:BCN2124
CAS No.:520-65-0
- Echimidine
Catalog No.:BCN1967
CAS No.:520-68-3
- Medroxyprogesterone
Catalog No.:BCC5231
CAS No.:520-85-4
- Isoschaftoside
Catalog No.:BCN3011
CAS No.:52012-29-0
- NCS-382
Catalog No.:BCC6794
CAS No.:520505-01-5
- H-Val-pNA
Catalog No.:BCC3139
CAS No.:52084-13-6
- 2-Hydroxy-7-O-methylscillascillin
Catalog No.:BCN5659
CAS No.:52096-50-1
- Mestanolone
Catalog No.:BCC9022
CAS No.:521-11-9
- Dromostanolone propionate
Catalog No.:BCC8954
CAS No.:521-12-0
- Androstenediol
Catalog No.:BCC8828
CAS No.:521-17-5
Sex differences and serotonergic mechanisms in the behavioural effects of psilocin.[Pubmed:26461483]
Behav Pharmacol. 2016 Jun;27(4):309-20.
Psilocybin has recently attracted a great deal of attention as a clinical research and therapeutic tool. The aim of this paper is to bridge two major knowledge gaps regarding its behavioural pharmacology - sex differences and the underlying receptor mechanisms. We used Psilocin (0.25, 1 and 4 mg/kg), an active metabolite of psilocybin, in two behavioural paradigms - the open-field test and prepulse inhibition (PPI) of the acoustic startle reaction. Sex differences were evaluated with respect to the phase of the female cycle. The contribution of serotonin receptors in the behavioural action was tested in male rats with selective serotonin receptor antagonists: 5-HT1A receptor antagonist (WAY100635 1 mg/kg), 5-HT2A receptor antagonist (MDL100907 0.5 mg/kg), 5-HT2B receptor antagonist (SB215505 1 mg/kg) and 5-HT2C receptor antagonist (SB242084 1 mg/kg). Psilocin induced dose-dependent inhibition of locomotion and suppression of normal behaviour in rats (behavioural serotonin syndrome, impaired PPI). The effects were more pronounced in male rats than in females. The inhibition of locomotion was normalized by 5-HT1A and 5-HT2B/C antagonists; however, PPI was not affected significantly by these antagonists. Our findings highlight an important issue of sex-specific reactions to Psilocin and that apart from 5-HT2A-mediated effects 5-HT1A and 5-HT2C/B receptors also play an important role. These findings have implications for recent clinical trials.
Metabolism of psilocybin and psilocin: clinical and forensic toxicological relevance.[Pubmed:28074670]
Drug Metab Rev. 2017 Feb;49(1):84-91.
Psilocybin and Psilocin are controlled substances in many countries. These are the two main hallucinogenic compounds of the "magic mushrooms" and both act as agonists or partial agonists at 5-hydroxytryptamine (5-HT)2A subtype receptors. During the last few years, psilocybin and Psilocin have gained therapeutic relevance but considerable physiological variability between individuals that can influence dose-response and toxicological profile has been reported. This review aims to discuss metabolism of psilocybin and Psilocin, by presenting all major and minor psychoactive metabolites. Psilocybin is primarily a pro-drug that is dephosphorylated by alkaline phosphatase to active metabolite Psilocin. This last is then further metabolized, Psilocin-O-glucuronide being the main urinary metabolite with clinical and forensic relevance in diagnosis.
DNA-based taxonomic identification of basidiospores in hallucinogenic mushrooms cultivated in "grow-kits" seized by the police: LC-UV quali-quantitative determination of psilocybin and psilocin.[Pubmed:27021629]
J Pharm Biomed Anal. 2016 Jun 5;125:427-32.
The taxonomic identification of the biological material contained in the hallucinogenic mushrooms culture media, was carried out using a DNA-based approach, thus highlighting the usefulness of this approach in the forensic identification of illegal samples also when they are present as basidiospores mixed in culture media and spore-bearing fruiting body are not present. This approach is very useful as it allows the unequivocal identification of potentially illicit material before the cultivation and it enables to stop the material to the Customs and to destroy it due to its dangerousness without cultivating the "grow-kits" and without instructing a criminal case. In fact, even if Psilocin and psilocybin and the whole mushrooms are illegal in many countries, there is no specific indication in the law about the so called "grow-kits", containing the spores. To confirm the data obtained by the taxonomic identification, a simple, reliable, efficient LC-UV method, using tryptamine as internal standard, suitable for the forensic quali-quantitative determination of Psilocin and psilocybin in hallucinogenic mushroom was optimized, validated and applied to the mushrooms grown after the cultivation of the grow-kits seized by the judicial authority, with the authorization of the Ministry of Health. A cation exchange column was used in a gradient elution mode (Phase A: 50mMK2HPO4; 100mM NaCl pH=3 Phase B: methanol). The developed method was linear over the calibration range with a R(2)>0.9992 for both the analytes. The detection and quantification limits were respectively 0.01 and 0.1mug/mL for psilocybin and 0.05mug/mL and 0.1mug/mL for Psilocin and the intra- and inter-day precision was satisfactory (coefficients of variation <2.0% for both the analytes). The content of psilocybin in the mushrooms grown from the seized "grow-kits" ranged from 1.02 to 7.60mg/g of dry vegetable material, while the content of Psilocin from 0.415 to 8.36mg/g.
Neurovascular and neuroimaging effects of the hallucinogenic serotonin receptor agonist psilocin in the rat brain.[Pubmed:26192543]
Neuropharmacology. 2015 Dec;99:210-20.
The development of pharmacological magnetic resonance imaging (phMRI) has presented the opportunity for investigation of the neurophysiological effects of drugs in vivo. Psilocin, a hallucinogen metabolised from psilocybin, was recently reported to evoke brain region-specific, phMRI signal changes in humans. The present study investigated the effects of Psilocin in a rat model using phMRI and then probed the relationship between neuronal and haemodynamic responses using a multimodal measurement preparation. Psilocin (2 mg/kg or 0.03 mg/kg i.v.) or vehicle was administered to rats (N=6/group) during either phMRI scanning or concurrent imaging of cortical blood flow and recording of local field potentials. Compared to vehicle controls Psilocin (2 mg/kg) evoked phMRI signal increases in a number of regions including olfactory and limbic areas and elements of the visual system. PhMRI signal decreases were seen in other regions including somatosensory and motor cortices. Investigation of neurovascular coupling revealed that whilst neuronal responses (local field potentials) to sensory stimuli were decreased in amplitude by Psilocin administration, concurrently measured haemodynamic responses (cerebral blood flow) were enhanced. The present findings show that Psilocin evoked region-specific changes in phMRI signals in the rat, confirming recent human data. However, the results also suggest that the haemodynamic signal changes underlying phMRI responses reflect changes in both neuronal activity and neurovascular coupling. This highlights the importance of understanding the neurovascular effects of pharmacological manipulations for interpreting haemodynamic neuroimaging data.
Contribution of a helix 5 locus to selectivity of hallucinogenic and nonhallucinogenic ligands for the human 5-hydroxytryptamine2A and 5-hydroxytryptamine2C receptors: direct and indirect effects on ligand affinity mediated by the same locus.[Pubmed:8700116]
Mol Pharmacol. 1996 Jul;50(1):34-42.
An important determinant of the neurobehavioral responses induced by a drug is its relative receptor selectivity. The molecular basis of ligand selectivity of hallucinogenic and nonhallucinogenic compounds of varying structural classes for the human 5-hydroxytryptamine (5-HT)2A and 5-HT2C receptors was investigated with the use of reciprocal site-directed mutagenesis. Because these two closely related receptor subtypes differ in the amino acid present at position 5.46 (residues 242 and 222 in the sequences, respectively), the effects of corresponding substitutions in the 5-HT2A[S5.46(242)-->A] and 5-HT2C[A5.46(222)-->S] receptors were studied in tandem. By studying both receptors, the direct and indirect effects of mutations on affinity and selectivity can be distinguished. The ergolines studied, mesulergine (selective for the 5-HT2C receptor) and d-lysergic acid diethylamide (selective for the 5-HT2A receptor), reversed their relative affinity with mutations in each receptor, supporting a direct role of this locus in the selectivity of these ligands. However, interchange mutations in either receptor led to decreased or unchanged affinity for (+/-)-1-)(2,5-dimethoxy-4-iodophenyl)-2-aminopropane and ketanserin, which have higher affinity for the 5-HT2A receptor, consistent with little contribution of this locus to the selectivity of these ligands. The indoleamines studied were affected differently by mutations in each receptor, suggesting that they bind differently to the two receptor subtypes. Mutation of this locus in the 5-HT2A receptor decreased the affinity of all indoleamines, whereas the interchange mutation of the 5-HT2C receptor did not affect indoleamine affinity. These results are consistent with a direct interaction between this side chain and indoleamines for the 5-HT2A receptor but not for the 5-HT2C receptor. Furthermore, this analysis shows that the higher affinity of 5-HT and tryptamine for the 5-HT2C receptor than for the 5-HT2A receptors is not due to the difference at this locus. The hallucinogens studied [d-lysergic acid diethylamide, Psilocin, bufotenin, and (+/-)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane] fell into different classes in this analysis. For the classes of ligand studied, the side-chain difference at this position directly determines relative ligand selectivity only for ergolines and may contribute to the specific effects of hallucinogens in this class.


