PriminCAS# 15121-94-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 15121-94-5 | SDF | Download SDF |
| PubChem ID | 84800 | Appearance | Yellow powder |
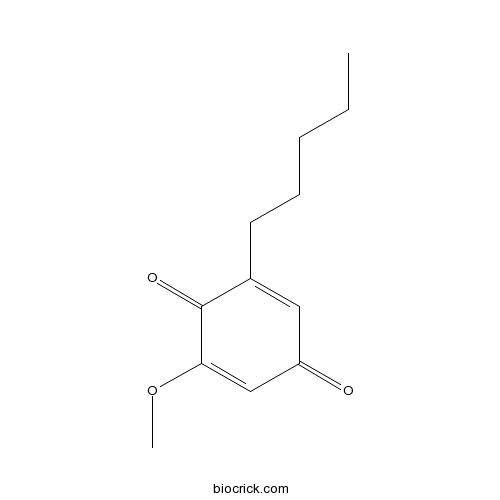
| Formula | C12H16O3 | M.Wt | 208.26 |
| Type of Compound | Quinones | Storage | Desiccate at -20°C |
| Solubility | Soluble in acetonitrile, ethanol, ethyl acetate and methan | ||
| Chemical Name | 2-methoxy-6-pentylcyclohexa-2,5-diene-1,4-dione | ||
| SMILES | CCCCCC1=CC(=O)C=C(C1=O)OC | ||
| Standard InChIKey | WLWIMKWZMGJRBS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H16O3/c1-3-4-5-6-9-7-10(13)8-11(15-2)12(9)14/h7-8H,3-6H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Primin has antimycobacterial, and strong antineoplastic actions. Primin has potent activity against Trypanosoma brucei rhodesiense (IC50 0.144 microM) and Leishmania donovani (IC50 0.711 microM), and low cytotoxicity (IC50 15.4 microM) on mammalian cells; it could serve as a lead compound for the rational design of more potent and less toxic antiprotozoal agents. |
| Targets | Antifection |
| In vitro | Antituberculotic and antiprotozoal activities of primin, a natural benzoquinone: in vitro and in vivo studies.[Pubmed: 17193236]Chem Biodivers. 2006 Nov;3(11):1230-7.Primin (=2-methoxy-6-pentylcyclohexa-2,5-diene-1,4-dione), a natural benzoquinone synthesized in our laboratory, was investigated for its in vitro antiprotozoal, antimycobacterial, and cytotoxic potential. |
| In vivo | First observations on the topical use of Primin, Plumbagin and Maytenin in patients with skin cancer.[Pubmed: 4620478]Rev Inst Antibiot (Recife). 1974 Dec;14(1-2):9-16.Eleven cases of patients bearing basic cellular carcinoma, and one case of patient bearing Kaposi's sarcomatosis, all treated with antibiotics isolated by Goncalves de Lima and Co-workers at the Instituto de Antibióticos, are presented by the authors. |
| Structure Identification | Med Chem. 2007 Jul;3(4):369-72.Synthesis and antitumour activity of the Primin (2-methoxy-6-n-pentyl-1,4-benzoquinone) and analogues.[Pubmed: 17627574]Cancer is a serious worldwide health threat, killing almost seven million people per year. Quinones are an important class of antitumour agents that are activated by tumour hypoxia. Primin (2-methoxy-6-n-pentyl-1,4-benzo-quinone), a naturally-occurring product obtained from Primula obconica (Primulaceae) has shown antimicrobial and antitumour properties. |

Primin Dilution Calculator

Primin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.8017 mL | 24.0085 mL | 48.0169 mL | 96.0338 mL | 120.0423 mL |
| 5 mM | 0.9603 mL | 4.8017 mL | 9.6034 mL | 19.2068 mL | 24.0085 mL |
| 10 mM | 0.4802 mL | 2.4008 mL | 4.8017 mL | 9.6034 mL | 12.0042 mL |
| 50 mM | 0.096 mL | 0.4802 mL | 0.9603 mL | 1.9207 mL | 2.4008 mL |
| 100 mM | 0.048 mL | 0.2401 mL | 0.4802 mL | 0.9603 mL | 1.2004 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Poricoic acid AM
Catalog No.:BCN8499
CAS No.:151200-92-9
- Borapetoside F
Catalog No.:BCN6413
CAS No.:151200-50-9
- Borapetoside E
Catalog No.:BCN6571
CAS No.:151200-49-6
- Borapetoside D
Catalog No.:BCN6612
CAS No.:151200-48-5
- 4'-Hydroxy-2,4-dimethoxychalcone
Catalog No.:BCC8708
CAS No.:151135-64-7
- CL 316243 disodium salt
Catalog No.:BCC7091
CAS No.:151126-84-0
- 8-(6-Hydroperoxy-3,7-dimethyl-2,7-octadienyloxy)psoralen
Catalog No.:BCN1558
CAS No.:151121-39-0
- 4-Difluoromethoxy-3-hydroxybenzaldehyde
Catalog No.:BCC8706
CAS No.:151103-08-1
- Haloperidol hydrochloride
Catalog No.:BCC4251
CAS No.:1511-16-6
- Moxifloxacin
Catalog No.:BCC4227
CAS No.:151096-09-2
- L-701,252
Catalog No.:BCC6755
CAS No.:151057-13-5
- DOXO-EMCH
Catalog No.:BCC1537
CAS No.:151038-96-9
- CP 135807
Catalog No.:BCC7774
CAS No.:151272-90-1
- Genkwanol C
Catalog No.:BCN8012
CAS No.:151283-11-3
- Inokosterone
Catalog No.:BCN3431
CAS No.:15130-85-5
- Zaleplon
Catalog No.:BCC5197
CAS No.:151319-34-5
- 3-O-p-Coumaroyloleanolic acid
Catalog No.:BCN3952
CAS No.:151334-06-4
- Ro 32-0432 hydrochloride
Catalog No.:BCC7122
CAS No.:151342-35-7
- Pseudolarolide B
Catalog No.:BCN8093
CAS No.:151368-43-3
- Estradiol hexahydrobenzoate
Catalog No.:BCC8962
CAS No.:15140-27-9
- Swietemahalactone
Catalog No.:BCN6886
CAS No.:1514669-21-6
- Ampelopsin F
Catalog No.:BCN3305
CAS No.:151487-08-0
- N-[[1-[(2-Nitrophenyl)sulfonyl]-1H-indole-3-yl]methyl]-N-[1-[1-[(2-nitrophenyl)sulfonyl]-1H-indole-3-yl]-2-oxo-2-(tert-butylamino)ethyl]-1-(2-diazo-3-oxobutyryl)-2-oxo-3-methylpiperidine-3beta-carboxamide
Catalog No.:BCC8335
CAS No.:151513-70-1
- Levomefolate calcium
Catalog No.:BCC1702
CAS No.:151533-22-1
[First observations on the topical use of Primin, Plumbagin and Maytenin in patients with skin cancer].[Pubmed:4620478]
Rev Inst Antibiot (Recife). 1974 Dec;14(1-2):9-16.
Eleven cases of patients bearing basic cellular carcinoma, and one case of patient bearing Kaposi's sarcomatosis, all treated with antibiotics isolated by Goncalves de Lima and Co-workers at the Instituto de Antibioticos, are presented by the authors. Primin, an antibiotic extracted from a vegetal named Miconia sp. (Herb. I.A.-1903) with a 2-metoxi-6-n-pentil-p benzoquinone structure, presented a strong antineoplastic action in the cases treated. Plumbagin isolated from Plumbago scandens in local use, was responsible for a complete healing of the injuries treated. Maytenin extracted from Maytenus sp. (Herb. I.A.-1750) showed less activity than the two previous mentioned, but with a low irritant action and late antineoplastic properties. The authors are going on these experiments. They believe that these antibodies, in local use, may advantageously substitute the surgery and the radiotherapy, meanly in those external ear tumidities and back of the nose, owing to a hurtful action in cartilage, provoked by radiotherapy.
Antituberculotic and antiprotozoal activities of primin, a natural benzoquinone: in vitro and in vivo studies.[Pubmed:17193236]
Chem Biodivers. 2006 Nov;3(11):1230-7.
Primin (=2-methoxy-6-pentylcyclohexa-2,5-diene-1,4-dione), a natural benzoquinone synthesized in our laboratory, was investigated for its in vitro antiprotozoal, antimycobacterial, and cytotoxic potential. Primin showed very potent activity against Trypanosoma brucei rhodesiense (IC50 0.144 microM) and Leishmania donovani (IC50 0.711 microM), and revealed low cytotoxicity (IC50 15.4 microM) on mammalian cells. Only moderate inhibitory activity was observed against Mycobacterium tuberculosis, Trypanosoma cruzi, and Plasmodium falciparum. When tested for in vivo efficacy in a Trypanosoma b. brucei rodent model, Primin failed to cure the infection at 20 mg/kg given intraperitoneally. Primin was too toxic in vivo at a higher concentration (30 mg/kg, injected i.p. route) in mice infected with L. donovani. Taken together, Primin can serve as a lead compound for the rational design of more potent and less toxic antiprotozoal agents.
Synthesis and antitumour activity of the Primin (2-methoxy-6-n-pentyl-1,4-benzoquinone) and analogues.[Pubmed:17627574]
Med Chem. 2007 Jul;3(4):369-72.
Cancer is a serious worldwide health threat, killing almost seven million people per year. Quinones are an important class of antitumour agents that are activated by tumour hypoxia. Primin (2-methoxy-6-n-pentyl-1,4-benzo-quinone), a naturally-occurring product obtained from Primula obconica (Primulaceae) has shown antimicrobial and antitumour properties. The synthesis of the Primin to obtain 3-, 5- or 6-alkyl substituted derivatives has been previously attempted seeking antitumour activity. The intermediate reaction products, 2-methoxy-hydroquinone-di-(2'-tetrahydro-pyranyl) ether and 2-methoxy-6-n-pentyl-hydroquinone-di-(2'-tetrahydropyranyl) ether were obtained and evaluated against sarcoma 180 (S-180) and Ehrlich carcinoma, as well as toxicity tests were performed. The antitumour activity tests showed that these intermediate compounds were able to inhibit S-180 sarcoma and Ehrlich carcinoma growth in mice. These results indicated that the tetrahydropyranyl protect group conserved the antitumour activity in comparison with quinone group, however, it exhibited a less toxic effect, with no characteristic of quinones. These results can suggest that compound 2-methoxy-6-n-pentyl-hydroquinone-di-(2'-tetrahydropyranyl) ether may act as a prodrug with some advantages in comparison with the Primin.


