PranoprofenCAS# 52549-17-4 |
- Dexpramipexole dihydrochloride
Catalog No.:BCC1528
CAS No.:104632-27-1
- Dexpramipexole
Catalog No.:BCC1527
CAS No.:104632-28-2
- Cariprazine hydrochloride
Catalog No.:BCC1454
CAS No.:1083076-69-0
- Cariprazine
Catalog No.:BCC1453
CAS No.:839712-12-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 52549-17-4 | SDF | Download SDF |
| PubChem ID | 4888 | Appearance | Powder |
| Formula | C15H13NO3 | M.Wt | 255.27 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (391.74 mM) *"≥" means soluble, but saturation unknown. | ||
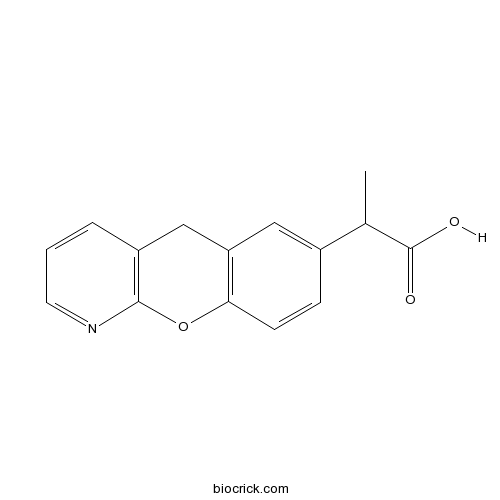
| Chemical Name | 2-(5H-chromeno[2,3-b]pyridin-7-yl)propanoic acid | ||
| SMILES | CC(C1=CC2=C(C=C1)OC3=C(C2)C=CC=N3)C(=O)O | ||
| Standard InChIKey | TVQZAMVBTVNYLA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H13NO3/c1-9(15(17)18)10-4-5-13-12(7-10)8-11-3-2-6-16-14(11)19-13/h2-7,9H,8H2,1H3,(H,17,18) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Pranoprofen is a non-steroidal anti-inflammatory drug used in ophthalmology.
Target: PGE2
Pranoprofen 0.1% was found to be as effective as diclofenac sodium 0.1% in reducing inflammation and pain after strabismus surgery. Pranoprofen could be used as a safe and effective anti-inflammatory alternative for the treatment of inflammation following strabismus surgery [1]. pranoprofen has efficacy equivalent to a moderate-potency corticosteroid with a better safety profile. It should be considered for the treatment of chronic conjunctivitis of presumed nonbacterial origin [2]. Pranoprofen inhibited ER stress-induced glucose regulated protein 78 (GRP78) expression, an ER-localized molecular chaperon. Moreover, pranoprofen inhibited ER stress-induced CCAAT/enhancer-binding protein homologous protein (CHOP) expression, an apoptotic transcription factor. the inhibitory effect of pranoprofen on ER stress-related genes (GRP78 and CHOP) would be mediated through the inhibition of XBP-1 splicing [3]. References: | |||||

Pranoprofen Dilution Calculator

Pranoprofen Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9174 mL | 19.5871 mL | 39.1742 mL | 78.3484 mL | 97.9355 mL |
| 5 mM | 0.7835 mL | 3.9174 mL | 7.8348 mL | 15.6697 mL | 19.5871 mL |
| 10 mM | 0.3917 mL | 1.9587 mL | 3.9174 mL | 7.8348 mL | 9.7936 mL |
| 50 mM | 0.0783 mL | 0.3917 mL | 0.7835 mL | 1.567 mL | 1.9587 mL |
| 100 mM | 0.0392 mL | 0.1959 mL | 0.3917 mL | 0.7835 mL | 0.9794 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Pranoprofen
- 5,7-Dihydroxychromone 7-rutinoside
Catalog No.:BCN3333
CAS No.:52538-46-2
- Quercetin 3-O-robinobioside
Catalog No.:BCN5676
CAS No.:52525-35-6
- Flavone
Catalog No.:BCN8477
CAS No.:525-82-6
- Kinetin
Catalog No.:BCC1679
CAS No.:525-79-1
- Harmalol
Catalog No.:BCC8183
CAS No.:525-57-5
- Fraxidin
Catalog No.:BCN5675
CAS No.:525-21-3
- Dihydrokainic acid
Catalog No.:BCC6556
CAS No.:52497-36-6
- PSB 36
Catalog No.:BCC7240
CAS No.:524944-72-7
- Peonidin-3-O-arabinoside chloride
Catalog No.:BCN3029
CAS No.:524943-91-7
- D-Tryptophanol
Catalog No.:BCC2699
CAS No.:52485-52-6
- 5-(Z-heptadec-8-enyl) resorcinol
Catalog No.:BCN5674
CAS No.:52483-19-9
- Isokurarinone
Catalog No.:BCN2890
CAS No.:52483-02-0
- Ingenol 3-palmitate
Catalog No.:BCN7686
CAS No.:52557-26-3
- bPiDDB
Catalog No.:BCC7606
CAS No.:525596-66-1
- Glycozolinine
Catalog No.:BCN5677
CAS No.:5257-08-9
- Acm-thiopropionic acid
Catalog No.:BCC2838
CAS No.:52574-08-0
- Phellamurin
Catalog No.:BCN5678
CAS No.:52589-11-4
- Neophellamuretin
Catalog No.:BCN7888
CAS No.:52589-20-5
- Iriflophenone
Catalog No.:BCN5679
CAS No.:52591-10-3
- Eudesmin
Catalog No.:BCN6563
CAS No.:526-06-7
- Sesamolin
Catalog No.:BCN1289
CAS No.:526-07-8
- Abrine
Catalog No.:BCN2348
CAS No.:526-31-8
- Tryptophol
Catalog No.:BCN5681
CAS No.:526-55-6
- Isoretronecanol
Catalog No.:BCN1993
CAS No.:526-63-6
Biopharmaceutical profile of hydrogels containing pranoprofen-loaded PLGA nanoparticles for skin administration: In vitro, ex vivo and in vivo characterization.[Pubmed:26844786]
Int J Pharm. 2016 Mar 30;501(1-2):350-61.
Pranoprofen (PF)-loaded nanoparticles (PF-F1NPs and PF-F2NPs) have been formulated into blank hydrogels (HG_PF-F1NPs and HG_PF-F1NPs) or into hydrogels composed of 3% azone (HG_PF-F1NPs-Azone and HG_PF-F2NPs-Azone), as innovative strategy to improve the biopharmaceutical profile of the selected non-steroidal anti-inflammatory drug (Pranoprofen, PF) for topical application. The purpose of this approach has been to increase the contact of PF with the skin, improve its retention in deeper layers, thus enhancing its anti-inflammatory and analgesic effects. The physicochemical characterization of the developed hydrogels showed a non-Newtonian behaviour, typical of semi-solid formulations for skin administration, with sustained release profile. The results obtained from ex vivo skin human permeation and in vivo anti-inflammatory efficacy studies suggest that topical application of HG_PF-F2NPs has been more effective in the treatment of oedema on the skin' surface in comparison to other hydrogels. No signs of skin irritancy have been detected for all the semi-solid formulations containing 0% or 3% azone.
In vitro, ex vivo and in vivo characterization of PLGA nanoparticles loading pranoprofen for ocular administration.[Pubmed:27480398]
Int J Pharm. 2016 Sep 25;511(2):719-27.
Pranoprofen (PF) is a NSAID considered as a safe anti-inflammatory treatment for strabismus and/or cataract surgery. The drug has been formulated in poly (lactic/glycolic) acid (PLGA) nanoparticles (PF-F1NPs with cPF 1.5mg/mL, PF-F2NPs with cPF 1mg/mL) produced by solvent displacement technique and tested the in vitro cytotoxicity, ex vivo corneal permeation, in vivo ocular tolerance and in vivo anti-inflammatory efficacy of PF-F1NPs, PF-F2NPs, in comparison to eye drops conventional dosage form (Oftalar((R)), PF 1mg/mL) and free drug solution (PF dissolved in PBS, 1.5mg/mL). The mean particle size of both formulations was around 350nm, with polydispersity index below 0.1, and a net negative charge of -7.41mV and -8.5mV for PF-F1NPs and PF-F2NPs, respectively. Y-79 human retinoblastoma cell line was used to evaluate the cytotoxicity of PF-F1NPs and PF-F2NPs, which were compared to blank NPs and free drug solution (PF dissolved in PBS, 1.5mg/mL). Concentrations up to 75mug/mL exhibited no toxicity to Y-79 cells, whereas at 150mug/mL a decrease of about 80% on the cell viability was observed after exposing the cells to PF-F1NPs. When treating the Y-79 cells with concentrations of PF-F2NPs between 1mug/mL to 100mug/mL, the cell viability was similar to control values after 24h and 48h of exposure. An ex vivo corneal permeation study was carried out in New Zealand rabbits. A very similar profile has been observed for the permeation of PF through the cornea when administered as eye drops and as free drug solution, which was kept much lower in comparison to PF-NPs formulations. The permeated amount of PF from the PF-F1NPs was slightly smaller than from PF-F2NPs, attributed to the increase of viscosity of the formulations with the increase of cPVA concentration. New Zealand white rabbits were also used to evaluate the irritancy of PF-F1NPs and PF-F2NPs, which demonstrated to be well-tolerated to the eye (i.e. the mean total score (MTS) was 0). PF-F2NPs exhibited the highest QP (amounts of PF permeated in the cornea) and significantly reduced the ocular edema compared to the tested formulations. The QR (amounts of PF retained in the cornea) of the PF-F1NPs was greater than that obtained for PF-F2NPs.


