Pomolic acid 28-O-beta-D-glucopyranosyl esterCAS# 83725-24-0 |
Quality Control & MSDS
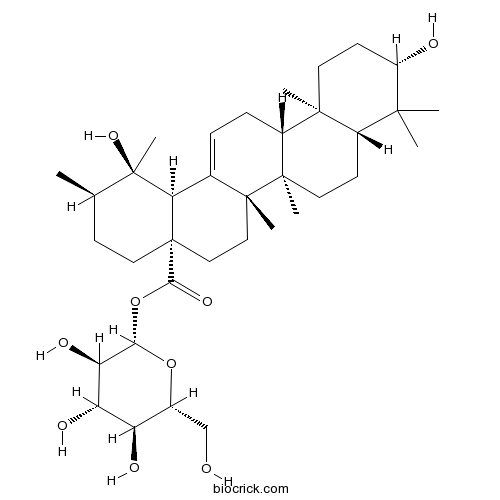
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 83725-24-0 | SDF | Download SDF |
| PubChem ID | 76322845 | Appearance | White powder |
| Formula | C36H58O9 | M.Wt | 634.9 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl] (1R,2R,4aS,6aR,6aS,6bR,8aR,10S,12aR,14bS)-1,10-dihydroxy-1,2,6a,6b,9,9,12a-heptamethyl-2,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylate | ||
| SMILES | CC1CCC2(CCC3(C(=CCC4C3(CCC5C4(CCC(C5(C)C)O)C)C)C2C1(C)O)C)C(=O)OC6C(C(C(C(O6)CO)O)O)O | ||
| Standard InChIKey | RRIMLWHUVCZACL-HPUCWRFUSA-N | ||
| Standard InChI | InChI=1S/C36H58O9/c1-19-10-15-36(30(42)45-29-27(41)26(40)25(39)21(18-37)44-29)17-16-33(5)20(28(36)35(19,7)43)8-9-23-32(4)13-12-24(38)31(2,3)22(32)11-14-34(23,33)6/h8,19,21-29,37-41,43H,9-18H2,1-7H3/t19-,21-,22+,23-,24+,25-,26+,27-,28-,29+,32+,33-,34-,35-,36+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Pomolic acid 28-O-beta-D-glucopyranosyl ester Dilution Calculator

Pomolic acid 28-O-beta-D-glucopyranosyl ester Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5751 mL | 7.8753 mL | 15.7505 mL | 31.501 mL | 39.3763 mL |
| 5 mM | 0.315 mL | 1.5751 mL | 3.1501 mL | 6.3002 mL | 7.8753 mL |
| 10 mM | 0.1575 mL | 0.7875 mL | 1.5751 mL | 3.1501 mL | 3.9376 mL |
| 50 mM | 0.0315 mL | 0.1575 mL | 0.315 mL | 0.63 mL | 0.7875 mL |
| 100 mM | 0.0158 mL | 0.0788 mL | 0.1575 mL | 0.315 mL | 0.3938 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ilexside I
Catalog No.:BCN3244
CAS No.:83725-19-3
- Dihydrosesamin
Catalog No.:BCN6616
CAS No.:83708-70-7
- (R)-AMPA
Catalog No.:BCC6582
CAS No.:83654-13-1
- RHC 80267
Catalog No.:BCC8083
CAS No.:83654-05-1
- CGRP (rat)
Catalog No.:BCC5712
CAS No.:83651-90-5
- (S)-AMPA
Catalog No.:BCC6583
CAS No.:83643-88-3
- Cyclosporin H
Catalog No.:BCC6448
CAS No.:83602-39-5
- Cropodine
Catalog No.:BCN2073
CAS No.:83601-85-8
- Regorafenib hydrochloride
Catalog No.:BCC1883
CAS No.:835621-07-3
- 2,2-Bis(3-amino-4-hydroxyphenyl)hexafluoropropane
Catalog No.:BCC8490
CAS No.:83558-87-6
- Anisofolin A
Catalog No.:BCN4377
CAS No.:83529-71-9
- Leucosceptoside A
Catalog No.:BCN7457
CAS No.:83529-62-8
- 3alpha-Acetoxy-20(29)-lupene-23,28-dioic acid
Catalog No.:BCN7508
CAS No.:83725-41-1
- Salvinorin A
Catalog No.:BCC5875
CAS No.:83729-01-5
- WH-4-023
Catalog No.:BCC8051
CAS No.:837422-57-8
- YM 244769
Catalog No.:BCC6222
CAS No.:837424-39-2
- Fmoc-Arg(Tos)-OH
Catalog No.:BCC3076
CAS No.:83792-47-6
- Fmoc-Ser(Bzl)-OH
Catalog No.:BCC3542
CAS No.:83792-48-7
- Falecalcitriol
Catalog No.:BCC1570
CAS No.:83805-11-2
- 2-Ethylhexyl trans-4-methoxycinnamate
Catalog No.:BCN1333
CAS No.:83834-59-7
- 4-Benzoyl 4'-methyldiphenyl sulfide
Catalog No.:BCC8694
CAS No.:83846-85-9
- Angeloylgomisin O
Catalog No.:BCN7361
CAS No.:83864-69-1
- Angeloylisogomisin O
Catalog No.:BCN4379
CAS No.:83864-70-4
- Obovatol
Catalog No.:BCN8265
CAS No.:83864-78-2
Glycosides of ursane-type triterpenoid, benzophenone, and iridoid from Vangueria agrestis (Fadogia agrestis) and their anti-infective activities.[Pubmed:30325205]
Nat Prod Res. 2018 Oct 16:1-9.
Four ursane-type triterpenoid glycosides (1-4), two benzophenone glycosides (5 and 6), and one iridoid glucoside (7) were isolated and characterized from the dried roots of Vangueria agrestis. Compounds 1 (3-O-[alpha-L-rhamnopyranosyl-(1-->2)-beta-D-xylopyranosyl]Pomolic acid 28-O-beta-D-glucopyranosyl ester) and 5 (2-O-[beta-D-apiofuranosyl-(1-->6)-beta-D-glucopyranosyl]-6,4'-dihydroxy-4-methox y benzophenone) were found to be new metabolites. The identity of all compounds has been accomplished, primarily, based on 1 D and 2 D NMR and HRESMS analysis. Compounds 6 and 2, showed inhibitory effect against Trypanosoma brucei with IC50 22.3 microM for 6 and IC50 11.1 microM, IC90 12.3 microM for 2.
1H and 13C NMR spectral data of new saponins from Cordia piauhiensis.[Pubmed:17559167]
Magn Reson Chem. 2007 Aug;45(8):692-4.
Two new bidesmoside triterpenoid saponins were isolated from stems of Cordia piauhiensis. Their structures, characterized as 3-O-alpha-L-rhamnopyranosyl-(1 --> 2)-beta-D-glucopyranosyl Pomolic acid 28-O-beta-D-glucopyranosyl ester (1) and 3-O-alpha-L-rhamnopyranosyl-(1 --> 2)-beta-D-glucopyranosyl oleanolic acid 28-O-beta-D-glucopyranosyl-(1 --> 6)-beta-D-glucopyranosyl ester (2), were unequivocally established after extensive NMR (1H, 13C, DEPT 135 degrees, COSY, HSQC, HMBC, TOCSY, and NOESY) studies.
Two new triterpenoid saponins from Sarcandra glabra.[Pubmed:16308199]
J Asian Nat Prod Res. 2005 Dec;7(6):829-34.
Two new triterpenoid saponins, named sarcandroside A and B, have been isolated from Sarcandra glabra (Thunb) Nakai. Their structures have been established as 3beta,19alpha,20beta-trihydroxyurs-11,13 (18)-diene-28,20beta-lactone-3-O-beta-D-glucopyranosyl (1 --> 3)-[alpha-L-rhamnopyranosyl(1 --> 2)]-beta-D-xylopyranoside (1) and 3-O-beta-D-glucopyranosyl (1 --> 3)-[alpha-L-rhamnopyranosyl(1 --> 2)]-beta-D-xylopyranosyl-Pomolic acid 28-O-beta-D-glucopyranosyl ester (2) by means of spectral and chemical methods.
Triterpenoids from Brazilian Ilex species and their in vitro antitrypanosomal activity.[Pubmed:15497942]
J Nat Prod. 2004 Oct;67(10):1697-700.
From the leaves of Ilex affinis and Ilex buxifolia, two adulterant species of "erva mate" (Ilex paraguariensis), three new triterpenoid glycosides were isolated. Affinoside 1 (3beta-O-[beta-D-glucopyranosyl-(1-->3)-[2-O-acetyl-(1-->2]]-alpha-L-arabinopyran osyl Pomolic acid 28-O-beta-D-glucopyranosyl ester, 1) was isolated from I. affinis, while buxifolioside I (28-O-beta-D-glucopyranosyl ester of (20S)-3alpha,19alpha-dihydroxyurs-12-ene-23,28-dioic acid, 7) and buxifolioside II (28-O-beta-D-glucopyranosyl ester of (20S)-3beta,19alpha-dihydroxyurs-12-en-24,28-dioic acid, 8) were isolated from I. buxifolia. Along with these new compounds, ilexoside II (2), ursolic acid (3), 28-nor-ursolic acid (4), 3beta-O-acetylursolic acid (5), and uvaol (6) were also isolated. The observed results confirm the structural specificity of the I. paraguariensis triterpenoids and reinforce a previous proposal to detect mate adulteration by triterpenoid analysis. In addition, the in vitro antitrypanosomal activity of some Ilex triterpenoids is also reported.


