PipermethystineCAS# 71627-22-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 71627-22-0 | SDF | Download SDF |
| PubChem ID | 194391 | Appearance | Powder |
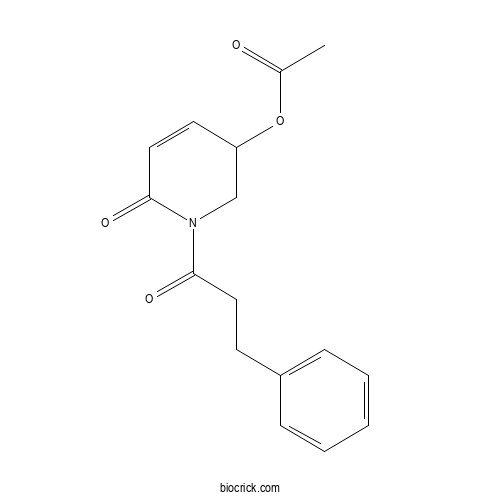
| Formula | C16H17NO4 | M.Wt | 287.3 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in acetone | ||
| Chemical Name | [6-oxo-1-(3-phenylpropanoyl)-2,3-dihydropyridin-3-yl] acetate | ||
| SMILES | CC(=O)OC1CN(C(=O)C=C1)C(=O)CCC2=CC=CC=C2 | ||
| Standard InChIKey | JLNNQCUATONMIT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H17NO4/c1-12(18)21-14-8-10-16(20)17(11-14)15(19)9-7-13-5-3-2-4-6-13/h2-6,8,10,14H,7,9,11H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Pipermethystine induces mitochondrial toxicity in human hepatoma cells, it is capable of causing cell death, probably in part by disrupting mitochondrial function, thus, it may contribute to rare but severe hepatotoxic reactions to kava. | |||||

Pipermethystine Dilution Calculator

Pipermethystine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4807 mL | 17.4034 mL | 34.8068 mL | 69.6136 mL | 87.0171 mL |
| 5 mM | 0.6961 mL | 3.4807 mL | 6.9614 mL | 13.9227 mL | 17.4034 mL |
| 10 mM | 0.3481 mL | 1.7403 mL | 3.4807 mL | 6.9614 mL | 8.7017 mL |
| 50 mM | 0.0696 mL | 0.3481 mL | 0.6961 mL | 1.3923 mL | 1.7403 mL |
| 100 mM | 0.0348 mL | 0.174 mL | 0.3481 mL | 0.6961 mL | 0.8702 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2',4'-Dihydroxydihydrochalcone
Catalog No.:BCN9946
CAS No.:53596-71-7
- Hyoscyamine hydrobromide
Catalog No.:BCN9945
CAS No.:306-03-6
- 1-Phenylethanol
Catalog No.:BCN9944
CAS No.:98-85-1
- 3',4',5'-Trimethoxyflavone
Catalog No.:BCN9943
CAS No.:67858-30-4
- Ethyl salicylate
Catalog No.:BCN9942
CAS No.:118-61-6
- Coniine hydrochloride
Catalog No.:BCN9941
CAS No.:15991-59-0
- Enterodiol
Catalog No.:BCN9940
CAS No.:77756-22-0
- Oleacein
Catalog No.:BCN9939
CAS No.:149183-75-5
- Apioline
Catalog No.:BCN9938
CAS No.:523-80-8
- Cevadine
Catalog No.:BCN9937
CAS No.:62-59-9
- Carvacrol methyl ether
Catalog No.:BCN9936
CAS No.:6379-73-3
- 2,4-Dimethylphenol
Catalog No.:BCN9935
CAS No.:105-67-9
- 2,4-Dihydroxybenzoic acid
Catalog No.:BCN9948
CAS No.:89-86-1
- Petroselinic acid
Catalog No.:BCN9949
CAS No.:593-39-5
- Manassantin B
Catalog No.:BCN9950
CAS No.:88497-88-5
- 4',5,7-Trihydroxy 3,3',6,8-tetramethoxyflavone
Catalog No.:BCN9951
CAS No.:58130-91-9
- 2',5,6',7-Tetrahydroxyflavone
Catalog No.:BCN9952
CAS No.:82475-00-1
- Lapatinib (GW-572016) Ditosylate
Catalog No.:BCN9953
CAS No.:388082-77-7
- Artepillin C
Catalog No.:BCN9954
CAS No.:72944-19-5
- 4-Methylcatechol
Catalog No.:BCN9955
CAS No.:452-86-8
- Isogentisin
Catalog No.:BCN9956
CAS No.:491-64-5
- p-Mentha-1,4-diene
Catalog No.:BCN9957
CAS No.:99-85-4
- Citronellyl acetate
Catalog No.:BCN9958
CAS No.:150-84-5
- Withanone
Catalog No.:BCN9959
CAS No.:27570-38-3
Transition-Metal-Free Total Synthesis and Revision of the Absolute Configuration of Pipermethystine.[Pubmed:31994875]
J Org Chem. 2020 Mar 6;85(5):3949-3953.
Starting from 3-hydroxy piperidines, a novel transition-metal-free strategy to 5-hydroxy-5,6-dihydro-2(1H)pyridones is reported. This unprecedented approach, which provides a practical, economical, and ecofriendly alternative to either the classical ring-closing metathesis of N-homoallyl-unsaturated amides or the dehydrogenation of amides, occurs by means of a triple C-H functionalization of three unreactive piperidine sp(3) carbons. The completion of the total synthesis revealed that the natural levo-isomer possesses the R absolute configuration, not S.
The direct and highly diastereoselective synthesis of 3,4-epoxy-2-piperidones. Application to the total synthesis and absolute configurational assignment of 3alpha,4alpha-epoxy-5beta-pipermethystine.[Pubmed:29192703]
Org Biomol Chem. 2017 Dec 19;16(1):77-88.
The substrate-controlled asymmetric total synthesis and absolute configurational assignment of biologically active 3alpha,4alpha-epoxy-5beta-Pipermethystine, a minor component in the aerial parts of kava, has been achieved by featuring, as a key step, the environmentally friendly and direct synthesis of 2,3-epoxyamides from allyl amines. By using the chiron approach, first a carbohydrate-derived dehydropiperidine was prepared and subjected to a stereoselective tandem C-H/C[double bond, length as m-dash]C oxidation reaction. In this attempt, the required alpha,alpha-trans-epoxy-2-piperidone skeleton of the kava metabolite precursor was not achieved, although the tandem oxidation was highly stereoselective. However, starting from non-carbohydrate 3-hydroxy-4,5-dehydropiperidine, and using the same tandem oxidation, the target intermediate was obtained in high yield and complete unprecedented anti-stereoselectivity. Since the proposed mechanistic course of this tandem oxidation implies the transient formation of an alpha,beta-unsaturated amide followed by the subsequent epoxidation reaction, this second approach supports the previously established biotransformation proposal of (-)-Pipermethystine to (-)-3alpha,4alpha-epoxy-5beta-Pipermethystine.
Are mould hepatotoxins responsible for kava hepatotoxicity?[Pubmed:22319018]
Phytother Res. 2012 Nov;26(11):1768-70.
Previous studies with kava components such as kavalactones, Pipermethystine and flavokavain B have demonstrated hepatotoxicity from these constituents. Regardless, there has recently been speculation that adulterants or impurities such as the mould hepatotoxin aflatoxin are a more likely cause of kava hepatotoxicity, despite a paucity of supporting evidence. Although there is limited similarity between acute kava hepatotoxicity and acute aflatoxicosis, and background levels of aflatoxin have been detected in kava samples, unless epidemiological investigations can uncover direct evidence implicating mould hepatotoxins, it remains more likely that chemical constituents of kava are the cause of the hepatotoxicity from kava.
Proposal for a kava quality standardization code.[Pubmed:21756963]
Food Chem Toxicol. 2011 Oct;49(10):2503-16.
Rare cases of hepatotoxicity emerged with the use of kava drugs and dietary supplements prepared from rhizomes and roots of the South Pacific plant kava (Piper methysticum). Their psychoactive, anxiolytic, relaxing, and recreational ingredients are the kavalactones kavain, dihydrokavain, methysticin, dihydromethysticin, yangonin, and desmethoxyyangonin, but there is little evidence that these kavalactones or the non-kavalactones Pipermethystine and flavokavain B are the culprits of the adverse hepatic reactions. It rather appears that poor quality of the kava material was responsible for the liver toxicity. Analysis of existing kava quality standardizations with focus on chemical, agricultural, manufacturing, nutritional, regulatory, and legislation backgrounds showed major shortcomings that could easily explain quality problems. We therefore suggest a uniform, internationally accepted device for kava quality standardizations that are in the interest of the consumers because of safety reasons and will meet the expectations of kava farmers, pharmaceutical manufacturers, regulators of agencies, and legislators. The initial step resides in the establishment of Pan-Pacific kava quality legislation as an important part of the proposed Kava Quality Standardization Code. In conclusion, a sophisticated approach to establish kava quality standardizations is needed for safe human use of kava as relaxing traditional beverages, the anxiolytic drugs, and recreational dietary supplements.
Constituents in kava extracts potentially involved in hepatotoxicity: a review.[Pubmed:21506562]
Chem Res Toxicol. 2011 Jul 18;24(7):992-1002.
Aqueous kava root preparations have been consumed in the South Pacific as an apparently safe ceremonial and cultural drink for centuries. However, several reports of hepatotoxicity have been linked to the consumption of kava extracts in Western countries, where mainly ethanolic or acetonic extracts are used. The mechanism of toxicity has not been established, although several theories have been put forward. The composition of the major constituents, the kava lactones, varies according to preparation method and species of kava plant, and thus, the toxicity of the individual lactones has been tested in order to establish whether a single lactone or a certain composition of lactones may be responsible for the increased prevalence of kava-induced hepatotoxicity in Western countries. However, no such conclusion has been made on the basis of current data. Inhibition or induction of the major metabolizing enzymes, which might result in drug interactions, has also gained attention, but ambiguous results have been reported. On the basis of the chemical structures of kava constituents, the formation of reactive metabolites has also been suggested as an explanation of toxicity. Furthermore, skin rash is a side effect in kava consumers, which may be indicative of the formation of reactive metabolites and covalent binding to skin proteins leading to immune-mediated responses. Reactive metabolites of kava lactones have been identified in vitro as glutathione (GSH) conjugates and in vivo as mercapturates excreted in urine. Addition of GSH to kava extracts has been shown to reduce cytotoxicity in vitro, which suggests the presence of inherently reactive constituents. Only a few studies have investigated the toxicity of the minor constituents present in kava extract, such as Pipermethystine and the flavokavains, where some have been shown to display higher in vitro cytotoxicity than the lactones. To date, there remains no indisputable reason for the increased prevalence of kava-induced hepatotoxicity in Western countries.
Kava and kava hepatotoxicity: requirements for novel experimental, ethnobotanical and clinical studies based on a review of the evidence.[Pubmed:21442674]
Phytother Res. 2011 Sep;25(9):1263-74.
Kava hepatotoxicity is a well described disease entity, yet there is uncertainty as to the culprit(s). In particular, there is so far no clear evidence for a causative role of kavalactones and non-kavalactone constituents, such as Pipermethystine and flavokavain B, identified from kava. Therefore, novel enzymatic, analytical, toxicological, ethnobotanical and clinical studies are now required. Studies should focus on the identification of further potential hepatotoxic constituents, considering in particular possible adulterants and impurities with special reference to ochratoxin A and aflatoxins (AFs) producing Aspergillus varieties, which should be urgently assessed and published. At present, Aspergillus and other fungus species producing hepatotoxic mycotoxins have not yet been examined thoroughly as possible contaminants of some kava raw materials. Its occurence may be facilitated by high humidity, poor methods for drying procedures and insufficient storage facilities during the time after harvest. Various experimental studies are recommended using aqueous, acetonic and ethanolic kava extracts derived from different plant parts, such as peeled rhizomes and peeled roots including their peelings, and considering both noble and non-noble kava cultivars. In addition, ethnobotanical studies associated with local expertise and surveillance are required to achieve a good quality of kava as the raw material. In clinical trials of patients with anxiety disorders seeking herbal anxiolytic treatment with kava extracts, long-term safety and efficacy should be tested using traditional aqueous extracts obtained from peeled rhizomes and peeled roots of a noble kava cultivar, such as Borogu, to evaluate the risk: benefit ratio. Concomitantly, more research should be conducted on the bioavailability of kavalactones and non-kavalactones derived from aqueous kava extracts. To be on the side of caution and to ensure lack of liver injury, kava consuming inhabitants of the kava producing or importing South Pacific islands should undergo assessment of their liver function values and serum aflatoxin levels. The primary aim is to achieve a good quality of kava raw material, without the risk of adulterants and impurities including ochratoxin A and AFs, which represent the sum of aflatoxin B1, B2, G1 and G2. Although it is known that kava may naturally be contaminated with AFs, there is at present no evidence that kava hepatotoxicity might be due to aflatoxicosis. However, appropriate studies have yet to be done and should be extended to other mould hepatotoxins, with the aim of publishing the obtained results. It is hoped that with the proposed qualifying measures, the safety of individuals consuming kava will substantially be improved.
Herbal hepatotoxicity by kava: update on pipermethystine, flavokavain B, and mould hepatotoxins as primarily assumed culprits.[Pubmed:21377431]
Dig Liver Dis. 2011 Sep;43(9):676-81.
Herbal hepatotoxicity by the anxiolytic kava (Piper methysticum Forst. f.) emerged unexpectedly and was observed in a few patients worldwide. Liver injury occurred after the use of traditional aqueous kava extracts in the South Pacific region and of acetonic and ethanolic extracts in Western countries in rare cases, suggesting that the solvents used play no major causative role. In this review, we discuss actual pathogenetic issues of kava hepatotoxicity with special focus on developments regarding Pipermethystine, flavokavain B, and mould hepatotoxins as possible culprits. There is abundant data of in vitro cytotoxicity including apoptosis by Pipermethystine and flavokavain B added to the incubation media, yet evidence is lacking of in vivo hepatotoxicity in experimental animals under conditions similar to human kava use. Furthermore, in commercial Western kava extracts, Pipermethystine was not detectable and flavokavain B was present as a natural compound in amounts much too low to cause experimental liver injury. There is concern, however, that due to high temperature and humidity in the South Pacific area, kava raw material might have been contaminated by mould hepatotoxins such as aflatoxins after harvest and during storage. Whether kava hepatotoxicity may be due to aflatoxicosis or other mould hepatotoxins, requires further studies.
Is the alkaloid pipermethystine connected with the claimed liver toxicity of Kava products?[Pubmed:18271308]
Pharmazie. 2008 Jan;63(1):71-4.
The pyridone alkaloid Pipermethystine has been considered to be responsible for alleged hepatoxicity of Kava products. Investigation of a series of retain samples of finished products from the German market and self-produced extracts from root and stem material of Piper methysticum clearly showed that Pipermethystine (1) is absent from all root and retain samples and extracts, with a limit of quantification of 45 ppm. As a positive control, leaves of P. methysticum showed an amount of 0.2% of 1. Thus, if there is any hepatotoxicity, compound 1 should not be the responsible constituent in the case reports with ethanolic extracts produced in Germany.
Effects of kava alkaloid, pipermethystine, and kavalactones on oxidative stress and cytochrome P450 in F-344 rats.[Pubmed:17329236]
Toxicol Sci. 2007 May;97(1):214-21.
Kava-containing products remain popular in the United States and continue to be sold in health food stores and ethnic markets regardless of the fact that it was banned in Western countries such as Germany, France, Switzerland, Australia, and Canada, following reports of alleged hepatotoxicity. It is therefore critical to establish efficacy and verify adverse effects and/or herb-drug interactions for kava-kava (Piper methysticum). We have previously demonstrated that kava alkaloid, Pipermethystine (PM), abundant in leaves and stem peelings, induces mitochondrial toxicity in human hepatoma cells, HepG2, as compared with the bioactive components, kavalactones (KL), abundant in the rhizome. The current study compared short-term toxic effects of PM in Fischer-344 (F-344) rats to acetone-water extracts of kava rhizome (KRE). Treatment of F-344 rats with PM (10 mg/kg) and KRE (100 mg/kg) for 2 weeks failed to elicit any significant changes in liver function tests or cause severe hepatic toxicity as measured by lipid peroxidation and apoptosis markers such as malondialdehyde, Bax, and Bcl-2. However, PM-treated rats demonstrated a significant increase in hepatic glutathione, cytosolic superoxide dismutase (Cu/ZnSOD), tumor necrosis factor alpha mRNA expression, and cytochrome P450 (CYP) 2E1 and 1A2, suggesting adaptation to oxidative stress and possible drug-drug interactions.
In vitro toxicity of kava alkaloid, pipermethystine, in HepG2 cells compared to kavalactones.[Pubmed:14737001]
Toxicol Sci. 2004 May;79(1):106-11.
Kava herbal supplements have been recently associated with acute hepatotoxicity, leading to the ban of kava products in approximately a dozen countries around the world. It is suspected that some alkaloids from aerial kava may have contributed to the problem. Traditionally, Pacific Islanders use primarily the underground parts of the shrub to prepare the kava beverage. However, some kava herbal supplements may contain ingredients from aerial stem peelings. The aim of this study was to test the in vitro effects of a major kava alkaloid, Pipermethystine (PM), found mostly in leaves and stem peelings, and kavalactones such as 7,8-dihydromethysticin (DHM) and desmethoxyyangonin (DMY), which are abundant in the roots. Exposure of human hepatoma cells, HepG2, to 100 microM PM caused 90% loss in cell viability within 24 h, while 50 microM caused 65% cell death. Similar concentrations of kavalactones did not affect cell viability for up to 8 days of treatment. Mechanistic studies indicate that, in contrast to kavalactones, PM significantly decreased cellular ATP levels, mitochondrial membrane potential, and induced apoptosis as measured by the release of caspase-3 after 24 h of treatment. These observations suggest that PM, rather than kavalactones, is capable of causing cell death, probably in part by disrupting mitochondrial function. Thus, PM may contribute to rare but severe hepatotoxic reactions to kava.
Piperidine alkaloids from Piper methysticum.[Pubmed:12711141]
Phytochemistry. 2003 May;63(2):193-8.
Pipermethystine (1), 3alpha,4alpha-epoxy-5beta-Pipermethystine (2) and awaine (3) were isolated from the aerial parts of kava (Piper methysticum G. Forster, Piperaceae) and identified by HRMS and NMR spectroscopic analysis. 1 was concentrated in the stem peelings and leaves. 2 and 3 are new alkaloids with 2 found only in cv. Isa among the 11 cultivars examined, and 3 occurred primarily in young leaves of all cultivars. The stem peelings have been used in recent years as a source of kavalactones in kava dietary supplement industry. Quantitative aspects of these piperidine alkaloids in P. methysticum and their potential activities on human physiology are discussed.
Facile enantiodivergent approach to 5-hydroxy-5,6-dihydro-2(1H)-pyridones. First total synthesis of both enantiomers of pipermethystine.[Pubmed:11594839]
Org Lett. 2001 Oct 18;3(21):3381-3.
[reaction: see text]. A novel enantiodivergent approach to 5-hydroxy-5,6-dihydro-2(1H)-pyridones using a ring closing metathesis and a lipase-mediated kinetic resolution as key steps is described and applied to the first synthesis of both enantiomers of Pipermethystine.


