3',4',5'-TrimethoxyflavoneCAS# 67858-30-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 67858-30-4 | SDF | Download SDF |
| PubChem ID | 332208 | Appearance | White-yellowish powder |
| Formula | C18H16O5 | M.Wt | 312.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in methan | ||
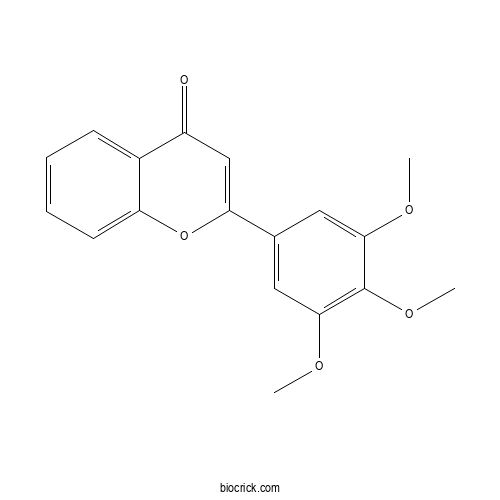
| Chemical Name | 2-(3,4,5-trimethoxyphenyl)chromen-4-one | ||
| SMILES | COC1=CC(=CC(=C1OC)OC)C2=CC(=O)C3=CC=CC=C3O2 | ||
| Standard InChIKey | QCXAJQVDUHKDEL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H16O5/c1-20-16-8-11(9-17(21-2)18(16)22-3)15-10-13(19)12-6-4-5-7-14(12)23-15/h4-10H,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | The 3',4',5-trimethoxyflavone derivative and its pharmaceutically acceptable salt inhibit corneal damage through excellent stimulatory action on mucus secretion in the conjunctiva and therefore may be effective as a prophylactic or therapeutic agent for dry eye syndrome. | |||||

3',4',5'-Trimethoxyflavone Dilution Calculator

3',4',5'-Trimethoxyflavone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.202 mL | 16.0102 mL | 32.0205 mL | 64.041 mL | 80.0512 mL |
| 5 mM | 0.6404 mL | 3.202 mL | 6.4041 mL | 12.8082 mL | 16.0102 mL |
| 10 mM | 0.3202 mL | 1.601 mL | 3.202 mL | 6.4041 mL | 8.0051 mL |
| 50 mM | 0.064 mL | 0.3202 mL | 0.6404 mL | 1.2808 mL | 1.601 mL |
| 100 mM | 0.032 mL | 0.1601 mL | 0.3202 mL | 0.6404 mL | 0.8005 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ethyl salicylate
Catalog No.:BCN9942
CAS No.:118-61-6
- Coniine hydrochloride
Catalog No.:BCN9941
CAS No.:15991-59-0
- Enterodiol
Catalog No.:BCN9940
CAS No.:77756-22-0
- Oleacein
Catalog No.:BCN9939
CAS No.:149183-75-5
- Apioline
Catalog No.:BCN9938
CAS No.:523-80-8
- Cevadine
Catalog No.:BCN9937
CAS No.:62-59-9
- Carvacrol methyl ether
Catalog No.:BCN9936
CAS No.:6379-73-3
- 2,4-Dimethylphenol
Catalog No.:BCN9935
CAS No.:105-67-9
- Nortropinone hydrochloride
Catalog No.:BCN9934
CAS No.:25602-68-0
- Sodium pyruvate
Catalog No.:BCN9933
CAS No.:113-24-6
- 3-Carene
Catalog No.:BCN9932
CAS No.:13466-78-9
- Pseudopelletierine hydrochloride
Catalog No.:BCN9931
CAS No.:6164-62-1
- 1-Phenylethanol
Catalog No.:BCN9944
CAS No.:98-85-1
- Hyoscyamine hydrobromide
Catalog No.:BCN9945
CAS No.:306-03-6
- 2',4'-Dihydroxydihydrochalcone
Catalog No.:BCN9946
CAS No.:53596-71-7
- Pipermethystine
Catalog No.:BCN9947
CAS No.:71627-22-0
- 2,4-Dihydroxybenzoic acid
Catalog No.:BCN9948
CAS No.:89-86-1
- Petroselinic acid
Catalog No.:BCN9949
CAS No.:593-39-5
- Manassantin B
Catalog No.:BCN9950
CAS No.:88497-88-5
- 4',5,7-Trihydroxy 3,3',6,8-tetramethoxyflavone
Catalog No.:BCN9951
CAS No.:58130-91-9
- 2',5,6',7-Tetrahydroxyflavone
Catalog No.:BCN9952
CAS No.:82475-00-1
- Lapatinib (GW-572016) Ditosylate
Catalog No.:BCN9953
CAS No.:388082-77-7
- Artepillin C
Catalog No.:BCN9954
CAS No.:72944-19-5
- 4-Methylcatechol
Catalog No.:BCN9955
CAS No.:452-86-8
Synthesis, biological evaluation, and NMR studies of 3-fluorinated derivatives of 3',4',5'-trihydroxyflavone and 3',4',5'-trimethoxyflavone.[Pubmed:33259925]
Bioorg Med Chem Lett. 2020 Nov 28;32:127720.
Flavones are valuable scaffolds in medicinal chemistry, especially as they display activity as antioxidants and neuroprotective agents. The need to incorporate a fluorine atom on flavones has driven much of the recent synthetic work in this area. We now report a route for the production of 3-fluorinated derivatives of 3',4',5'-trihydroxyflavone and 3',4',5'-trimethoxyflavone. Biological evaluation of these agents, along with their non-fluorinated counterparts, demonstrate that antioxidant activity may be enhanced whereas neuroprotective activity is conserved. Also, the 3-fluoro-3',4',5'-trihydroxyflavone can act as an NMR probe to detect structural changes during its action as a radical scavenger.
Biotransformation of Methoxyflavones by Selected Entomopathogenic Filamentous Fungi.[Pubmed:32854359]
Int J Mol Sci. 2020 Aug 25;21(17). pii: ijms21176121.
The synthesis and biotransformation of five flavones containing methoxy substituents in the B ring: 2'-, 3'-, 4'-methoxyflavones, 2',5'-dimethoxyflavone and 3',4',5'-trimethoxyflavone are described. Strains of entomopathogenic filamentous fungi were used as biocatalysts. Five strains of the species Beauveria bassiana (KCh J1.5, J2.1, J3.2, J1, BBT), two of the species Beauveria caledonica (KCh J3.3, J3.4), one of Isaria fumosorosea (KCh J2) and one of Isaria farinosa (KCh KW 1.1) were investigated. Both the number and the place of attachment of the methoxy groups in the flavonoid structure influenced the biotransformation rate and the amount of nascent products. Based on the structures of products and semi-products, it can be concluded that their formation is the result of a cascading process. As a result of enzymes produced in the cells of the tested strains, the test compounds undergo progressive demethylation and/or hydroxylation and 4-O-methylglucosylation. Thirteen novel flavonoid 4-O-methylglucosides and five hydroxy flavones were isolated and identified.
Cytotoxic activities of flavonoids from Centaurea scoparia.[Pubmed:25114960]
ScientificWorldJournal. 2014;2014:274207.
Phytochemical studies on the ethanolic extract of the aerial parts of Centaurea scoparia led to the isolation of two new flavonoids, 3',4'-dihydroxy-(3'',4''-dihydro-3''-hydroxy-4''-acetoxy)-2'',2''-dimethylpyrano- (5'',6'':7,8)-flavone-3-O- beta -D-glucopyranoside (1) and 3,3',4'-trihydroxy-(3'',4''-dihydro-3'',4''-dihydroxy)-2'',2''-dimethylpyrano-(5' ',6'':7,8)-flavone (2), along with eight known flavonoids isolated for the first time from this plant, cynaroside (3), Apigetrin (4), centaureidin (5), oroxylin A (6), 5,7-dihydroxy-3',4',5'-trimethoxyflavone (7), atalantoflavone (8), 5-hydroxy-3',4',8-trimethoxy-2'',2''-dimethylpyrano (5'',6'':6,7)-flavone (9), and 3',4',5,8-tetramethoxy-2'',2''-dimethylpyrano (5'',6'':6,7)-flavone (10). The structures of the isolated compounds were elucidated by means of spectroscopic tools including 1D and 2D NMR, UV, IR, and mass spectroscopy. Cytotoxic activities of the isolated compounds were evaluated against human cervical carcinoma HeLa, human hepatocellular carcinoma HepG2, and human breast carcinoma MCF-7. Compound 2 was the most potent cytotoxic agent against HeLa cells with an IC50 0.079 muM.
Two new flavonoid glycosides from Artemisia frigida Willd.[Pubmed:21061216]
J Asian Nat Prod Res. 2010 Nov;12(11):950-4.
An investigation of the n-BuOH-soluble fraction from the aerial parts of Artemisia frigida has led to the isolation of two new flavonoid glycosides, named friginoside A and friginoside B. Their structures were characterized as 5,7-dihydroxy-3',4',5'-trimethoxy flavone 7-O-beta-d-glucuronide (1) and 5,7-dihydroxy-3',4',5'-trimethoxyflavone 7-O-beta-d-glucuronyl-(1 --> 2)O-beta-d-glucuronide (2) on the basis of 1D and 2D NMR spectral analysis.
Constituents of Vittaria anguste-elongata and their biological activities.[Pubmed:16124757]
J Nat Prod. 2005 Aug;68(8):1180-4.
Twelve new compounds, vittarin-A (1), -B (2), -C (3), -D (4), -E (5), -F (6), 3-O-acetylniduloic acid (7), ethyl 3-O-acetylniduloate (8), methyl 4-O-coumaroylquinate (9), vittarilide-A (10), and -B (11), and vittariflavone (12), as well as 20 known compounds have been isolated from the whole plant of Vittaria anguste-elongata. The structures of these compounds were determined by spectroscopic and chemical transformation methods. 5,7-Dihydroxy-3',4',5'-trimethoxyflavone (18) displayed moderate cytotoxicity against human lung carcinoma and central nervous system carcinoma cell lines with inhibition of 89 and 61% at a concentration of 58 microM, respectively. Vittarilide-A (10) and -B (11) and ethyl 4-O-caffeoylquinate (14) exhibited moderate DPPH radical scavenging activity with IC50 values of 91, 290, and 234 microM, respectively.
Lipophilic flavones of Primula veris L. from field cultivation and in vitro cultures.[Pubmed:15896373]
Phytochemistry. 2005 May;66(9):1033-9.
Ten lipophilic flavones were isolated from the leaves of Primula veris from field cultivation - the newly described 3'-hydroxy-4',5'-dimethoxyflavone and 3'-methoxy-4',5'-methylenedioxyflavone, the previously known from chemical synthesis 3',4'-dimethoxyflavone, 2',5'-dimethoxyflavone, and also flavone, 2'-hydroxyflavone, 2'-methoxyflavone, 3'-methoxyflavone, 3',4',5'-trimethoxyflavone and 5,6,2',6'-tetramethoxyflavone (zapotin) which were previously known from plants. The same flavones were found in the leaves of P. veris obtained by in vitro propagation. The structural assignments were derived from (1)H NMR, (13)C NMR, EIMS and UV spectral data and the influence of B-ring oxygen substituents on the C-2, C-3 and H-3 NMR resonances in flavones unsubstituted in the A ring is taken into consideration.
Evaluation of detection methods for the reversed-phase HPLC determination of 3',4',5'-trimethoxyflavone in different phytopharmaceutical products and in human serum.[Pubmed:11705238]
Phytochem Anal. 2001 Mar-Apr;12(2):104-9.
Quantitative determination of the major compound, 3',4',5'-trimethoxyflavone (1), in plant extracts, in tablets of Flos and of Radix Primulae veris and in human serum has been accomplished using reversed-phase HPLC with UV, fluorescence and mass spectrometric (MS) detection. Compared to UV detection, fluorescence detection showed greater selectivity, was 10-fold more sensitive and allowed the determination of 1 in human serum after sample pre-treatment by solid-phase extraction. MS detection of 1 using electrospray ionisation (ESI) interface could be improved by substituting trifluoroacetic acid with the more polar and less conductive additive acetic acid, giving rise to a 230-fold improvement in analyte detectability at the cost of an increase of only 45% in the peak width of the eluting peak at its half height. Further optimisation of the acetic acid concentration showed the highest signal intensity at 1.25% for HPLC-atmospheric pressure chemical ionisation (APCI)-MS and at 0.75% for HPLC-ESI-MS. The optimised MS method proved to be extremely selective, 50 times more sensitive than UV detection and 5 times more sensitive than fluorescence detection. Furthermore, fragment-ion spectra produced by collision induced dissociation-MS have been used as "fingerprints" for identifying compounds in the highly complex mixtures examine.
A new flavone O-glycoside and other constituents from wheat leaves (Triticum aestivum L.).[Pubmed:10928543]
Z Naturforsch C J Biosci. 2000 May-Jun;55(5-6):337-40.
From leaves of Triticum aestivum a new O-glycosylflavone has been isolated together with chlorogenic acid and its 3'-methyl ether and 6 C-glycosylflavones. The structure of the new flavonoid was determined by 1D and 2D NMR techniques and other spectral evidence as 5,7-dihydroxy-3',4',5'-trimethoxyflavone-7-O-beta-rutinoside.
Metabolism of myricetin and related compounds in the rat. Metabolite formation in vivo and by the intestinal microflora in vitro.[Pubmed:4655415]
Biochem J. 1972 Nov;130(1):141-51.
1. The metabolism of a group of polyphenols related in structure to myricetin (3,5,7,3',4',5'-hexahydroxyflavone), including myricetin, myricitrin, 3,4,5-trihydroxyphenylacetic acid, delphinidin, robinetin, tricetin, tricin, malvin and 5,7-dihydroxy-3',4',5'-trimethoxyflavone, has been studied both in vivo after oral administration to the rat and in vitro in cultures of micro-organisms derived from the intestine of the rat. 2. It was shown that the rat intestinal microflora are able to degrade compounds of this group to the ring-fission products observed in urine after oral administration of the specific flavonoid. 3. All flavones and flavonols possessing free 5- and 7-hydroxyl groups in the A ring and a free 4'-hydroxyl group in the B ring gave rise to ring-fission products that included 3',5'-dihydroxyphenylacyl derivatives. 4. The metabolites 3,5-dihydroxyphenylacetic acid, 3-hydroxyphenylacetic acid, 3,5-dihydroxyphenylpropionic acid and 3-hydroxyphenylpropionic acid were isolated and identified by chromatographic and spectral methods. 5. On anaerobic incubation in a thioglycollate medium it was shown that intestinal micro-organisms can effect cleavage of glycosidic bonds, ring fission of certain flavonoid molecules showing 3',4',5'-trihydroxyphenyl substitution and dehydroxylation of certain flavonoid metabolites. 6. The urinary excretion of the metabolites 3,5-dihydroxyphenylacetic acid and 3-hydroxyphenylacetic acid was completely abolished when neomycin-treated rats were used.


