PicrinineCAS# 4684-32-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 4684-32-6 | SDF | Download SDF |
| PubChem ID | 46229104 | Appearance | Powder |
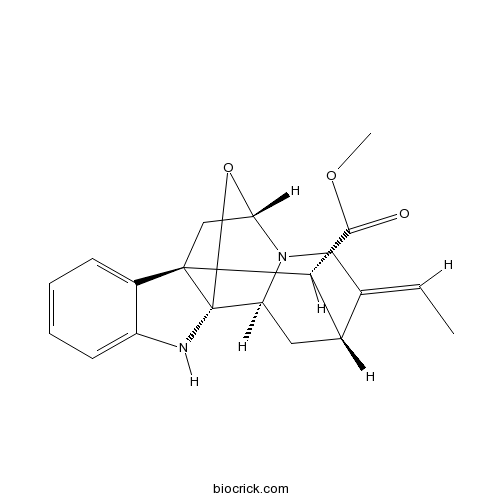
| Formula | C20H22N2O3 | M.Wt | 338.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | methyl (1R,9R,11S,14E,15R,17S,19R)-14-ethylidene-18-oxa-2,12-diazahexacyclo[9.6.1.19,15.01,9.03,8.012,17]nonadeca-3,5,7-triene-19-carboxylate | ||
| SMILES | CC=C1CN2C3CC1C(C45C3(NC6=CC=CC=C64)OC2C5)C(=O)OC | ||
| Standard InChIKey | BDXYPHKGNUGUFG-VETGLWQVSA-N | ||
| Standard InChI | InChI=1S/C20H22N2O3/c1-3-11-10-22-15-8-12(11)17(18(23)24-2)19-9-16(22)25-20(15,19)21-14-7-5-4-6-13(14)19/h3-7,12,15-17,21H,8-10H2,1-2H3/b11-3-/t12-,15-,16-,17-,19-,20-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Picrinine has notable antitussive, antiasthmatic, expectorant, antiinflammatory and analgesic effects; medicinal composition containing picrinine as active ingredient can be prepared into multiple dosage forms and can be used for treating cold, fever, and respiratory disease caused by fever with good therapeutic effect, convenient application and high safety; picrinine can be used as quality index for pharmic control in preparing medicine for treating cold, fever and respiratory disease caused by fever. |

Picrinine Dilution Calculator

Picrinine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9551 mL | 14.7754 mL | 29.5508 mL | 59.1017 mL | 73.8771 mL |
| 5 mM | 0.591 mL | 2.9551 mL | 5.9102 mL | 11.8203 mL | 14.7754 mL |
| 10 mM | 0.2955 mL | 1.4775 mL | 2.9551 mL | 5.9102 mL | 7.3877 mL |
| 50 mM | 0.0591 mL | 0.2955 mL | 0.591 mL | 1.182 mL | 1.4775 mL |
| 100 mM | 0.0296 mL | 0.1478 mL | 0.2955 mL | 0.591 mL | 0.7388 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Norscopolamine
Catalog No.:BCN3983
CAS No.:4684-28-0
- Orphenadrine Citrate
Catalog No.:BCC4572
CAS No.:4682-36-4
- Drimenol
Catalog No.:BCN7224
CAS No.:468-68-8
- Mesembrenone
Catalog No.:BCN3753
CAS No.:468-54-2
- Lupulon
Catalog No.:BCC8204
CAS No.:468-28-0
- Colupulone
Catalog No.:BCN8097
CAS No.:468-27-9
- Lu AE58054
Catalog No.:BCC1707
CAS No.:467459-31-0
- Lu AE58054 Hydrochloride
Catalog No.:BCC1708
CAS No.:467458-02-2
- Nootkatone
Catalog No.:BCN5517
CAS No.:4674-50-4
- Diphenyleneiodonium chloride
Catalog No.:BCC6670
CAS No.:4673-26-1
- 17-DMAG (Alvespimycin) HCl
Catalog No.:BCC1175
CAS No.:467214-21-7
- Alvespimycin
Catalog No.:BCC1346
CAS No.:467214-20-6
- Dihydrocorynantheine
Catalog No.:BCN3747
CAS No.:4684-43-9
- 3-Benzofurancarboxaldehyde
Catalog No.:BCC8622
CAS No.:4687-25-6
- Cimilactone A
Catalog No.:BCN7948
CAS No.:468733-06-4
- BMS-536924
Catalog No.:BCC1177
CAS No.:468740-43-4
- Hamamelitannin
Catalog No.:BCC8182
CAS No.:469-32-9
- Cycloeucalenol
Catalog No.:BCN5519
CAS No.:469-39-6
- Jervine
Catalog No.:BCN2975
CAS No.:469-59-0
- 5'-IMPdisodium salt
Catalog No.:BCN8175
CAS No.:4691-65-0
- Carbenicillin
Catalog No.:BCC5192
CAS No.:4697-36-3
- Uncarine D
Catalog No.:BCC8262
CAS No.:4697-68-1
- Isoalantolactone
Catalog No.:BCN4955
CAS No.:470-17-7
- Cinobufagin
Catalog No.:BCN5367
CAS No.:470-37-1
Total synthesis of the akuammiline alkaloid picrinine.[Pubmed:24597784]
J Am Chem Soc. 2014 Mar 26;136(12):4504-7.
We report the first total synthesis of the complex akuammiline alkaloid Picrinine, which was first isolated nearly five decades ago. Our synthetic approach features a concise assembly of the [3.3.1]-azabicyclic core, a key Fischer indolization reaction to forge the natural product's carbon framework, and a series of delicate late-stage transformations to complete the synthesis. Our synthesis of Picrinine also constitutes a formal synthesis of the related polycyclic alkaloid strictamine.
Fischer Indolizations as a Strategic Platform for the Total Synthesis of Picrinine.[Pubmed:26134260]
J Org Chem. 2015 Sep 18;80(18):8954-67.
Picrinine, which is a member of the akuammiline family of alkaloids, was first isolated in 1965 from the leaves of Alstonia scholaris. The natural product possesses a daunting polycyclic skeleton that contains a furanoindoline, a bridged [3.3.1]azabicycle, two N,O-acetal linkages, and six stereogenic centers. These structural features render Picrinine a challenging and attractive target for total synthesis. This paper provides a full account of our synthetic forays toward Picrinine, which culminates in the first total synthesis of this long-standing target. Central to the success of our approach is the use of the Fischer indolization reaction to introduce the C7 quaternary stereocenter and the indoline nucleus of the natural product's scaffold. We probe some of the subtleties of this classic transformation by examining some of the most complex Fischer indolization substrates to date. Additionally, we describe various roadblocks encountered in our experimental efforts, which were successfully overcome to complete the total synthesis of Picrinine.


