DrimenolCAS# 468-68-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 468-68-8 | SDF | Download SDF |
| PubChem ID | 3080551 | Appearance | Cryst. |
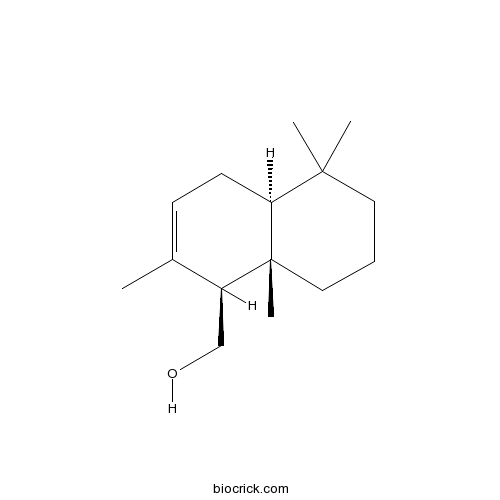
| Formula | C15H26O | M.Wt | 222.37 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(1S,4aS,8aS)-2,5,5,8a-tetramethyl-1,4,4a,6,7,8-hexahydronaphthalen-1-yl]methanol | ||
| SMILES | CC1=CCC2C(CCCC2(C1CO)C)(C)C | ||
| Standard InChIKey | HMWSKUKBAWWOJL-KCQAQPDRSA-N | ||
| Standard InChI | InChI=1S/C15H26O/c1-11-6-7-13-14(2,3)8-5-9-15(13,4)12(11)10-16/h6,12-13,16H,5,7-10H2,1-4H3/t12-,13-,15+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Drimenol shows antifungal activity, it is able to affect Botrytis cinerea growth with the EC50 value of 80 ppm. Drimendiol also shows quorum sensing inhibition activity. |
| Targets | Antifection |
| In vitro | Effect of drimenol and synthetic derivatives on growth and germination of Botrytis cinerea: Evaluation of possible mechanism of action.[Pubmed: 28911740]Pestic Biochem Physiol. 2017 Sep;141:50-56.The aim of this study was to determine the antifungal activity of Drimenol (1) and its synthetic derivatives, nordrimenone (2), drimenyl acetate (3), and drimenyl-epoxy-acetate (4), and to establish a possible mechanism of action for Drimenol. Drimendiol, a drimane sesquiterpene with quorum sensing inhibition activity.[Pubmed: 23513712]Nat Prod Commun. 2013 Feb;8(2):147-8.Quorum sensing (QS) is a regulatory mechanism that enables bacteria to make collective decisions such as an increase in virulence factors and biofilm production. Inhibitors of QS are important research tools in the discovery of new potential anti-bacterial agents. |

Drimenol Dilution Calculator

Drimenol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.497 mL | 22.485 mL | 44.9701 mL | 89.9402 mL | 112.4252 mL |
| 5 mM | 0.8994 mL | 4.497 mL | 8.994 mL | 17.988 mL | 22.485 mL |
| 10 mM | 0.4497 mL | 2.2485 mL | 4.497 mL | 8.994 mL | 11.2425 mL |
| 50 mM | 0.0899 mL | 0.4497 mL | 0.8994 mL | 1.7988 mL | 2.2485 mL |
| 100 mM | 0.045 mL | 0.2249 mL | 0.4497 mL | 0.8994 mL | 1.1243 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Mesembrenone
Catalog No.:BCN3753
CAS No.:468-54-2
- Lupulon
Catalog No.:BCC8204
CAS No.:468-28-0
- Colupulone
Catalog No.:BCN8097
CAS No.:468-27-9
- Lu AE58054
Catalog No.:BCC1707
CAS No.:467459-31-0
- Lu AE58054 Hydrochloride
Catalog No.:BCC1708
CAS No.:467458-02-2
- Nootkatone
Catalog No.:BCN5517
CAS No.:4674-50-4
- Diphenyleneiodonium chloride
Catalog No.:BCC6670
CAS No.:4673-26-1
- 17-DMAG (Alvespimycin) HCl
Catalog No.:BCC1175
CAS No.:467214-21-7
- Alvespimycin
Catalog No.:BCC1346
CAS No.:467214-20-6
- Theaflavin
Catalog No.:BCN5419
CAS No.:4670-05-7
- Rehmannic acid
Catalog No.:BCN4632
CAS No.:467-81-2
- Coronaridine
Catalog No.:BCN3762
CAS No.:467-77-6
- Orphenadrine Citrate
Catalog No.:BCC4572
CAS No.:4682-36-4
- Norscopolamine
Catalog No.:BCN3983
CAS No.:4684-28-0
- Picrinine
Catalog No.:BCN5518
CAS No.:4684-32-6
- Dihydrocorynantheine
Catalog No.:BCN3747
CAS No.:4684-43-9
- 3-Benzofurancarboxaldehyde
Catalog No.:BCC8622
CAS No.:4687-25-6
- Cimilactone A
Catalog No.:BCN7948
CAS No.:468733-06-4
- BMS-536924
Catalog No.:BCC1177
CAS No.:468740-43-4
- Hamamelitannin
Catalog No.:BCC8182
CAS No.:469-32-9
- Cycloeucalenol
Catalog No.:BCN5519
CAS No.:469-39-6
- Jervine
Catalog No.:BCN2975
CAS No.:469-59-0
- 5'-IMPdisodium salt
Catalog No.:BCN8175
CAS No.:4691-65-0
- Carbenicillin
Catalog No.:BCC5192
CAS No.:4697-36-3
Identification of a drimenol synthase and drimenol oxidase from Persicaria hydropiper, involved in the biosynthesis of insect deterrent drimanes.[Pubmed:28258968]
Plant J. 2017 Jun;90(6):1052-1063.
The sesquiterpenoid polygodial, which belongs to the drimane family, has been shown to be an antifeedant for a number of herbivorous insects. It is presumed to be synthesized from farnesyl diphosphate via Drimenol, subsequent C-12 hydroxylation and further oxidations at both C-11 and C-12 to form a dialdehyde. Here, we have identified a Drimenol synthase (PhDS) and a cytochrome P450 Drimenol oxidase (PhDOX1) from Persicaria hydropiper. Expression of PhDS in yeast and plants resulted in production of Drimenol alone. Co-expression of PhDS with PhDOX1 in yeast yielded drimendiol, the 12-hydroxylation product of Drimenol, as a major product, and cinnamolide. When PhDS and PhDOX1 were transiently expressed by agro-infiltration in Nicotiana benthamiana leaves, Drimenol was almost completely converted into cinnamolide and several additional Drimenol derivatives were observed. In vitro assays showed that PhDOX1 only catalyses the conversion from Drimenol to drimendiol, and not the further oxidation into an aldehyde. In yeast and heterologous plant hosts, the C-12 position of drimendiol is therefore likely to be further oxidized by endogenous enzymes into an aldehyde and subsequently converted to cinnamolide, presumably by spontaneous hemiacetal formation with the C-11 hydroxyl group followed by oxidation. Purified cinnamolide was confirmed by NMR and shown to be deterrent with an effective deterrent dose (ED50 ) of about 200-400 mug g(-1) fresh weight against both whiteflies and aphids. The putative additional physiological and biochemical requirements for polygodial biosynthesis and stable storage in plant tissues are discussed.
Study on the cytotoxic activity of drimane sesquiterpenes and nordrimane compounds against cancer cell lines.[Pubmed:25412045]
Molecules. 2014 Nov 18;19(11):18993-9006.
Twelve drimanes, including polygodial (1), isopolygodial (2), Drimenol (3), confertifolin (4), and isodrimenin (5), were obtained from natural sources. Semi-synthetic derivatives 6-12 were obtained from 1 and 2, and cytotoxic activity was evaluated in vitro against cancer cell lines (HT-29, MDA-MB231, DHF, MCF-7, PC-3, DU-145, and CoN). IC50 values were determined at concentrations of 12.5-100 microM of each compound for 72 h. In addition, it was found that polygodial (1), 8, and 12 induced changes in mitochondrial membrane permeability in CoN, MCF-7, and PC-3 cells.
Practical isolation of polygodial from Tasmannia lanceolata: a viable scaffold for synthesis.[Pubmed:26377594]
Org Biomol Chem. 2015 Dec 14;13(46):11200-7.
Polygodial, a valuable sesquiterpene dialdehyde featuring an epimerizable stereocenter was efficiently extracted and isolated in gram-scale quantities (3.3% w/w) from Tasmannia lanceolata (Tasmanian native pepper) via a recently developed rapid pressurised hot water extraction (PHWE) technique that utilises an unmodified household espresso machine. This method was compared to the maceration of T. lanceolata under a range of conditions. Polygodial was used to achieve semi-syntheses of closely related sesquiterpene natural products drimendiol, (-)-Drimenol, (+)-euryfuran, and some non-natural derivatives.
Molecular cloning and characterization of drimenol synthase from valerian plant (Valeriana officinalis).[Pubmed:25447532]
FEBS Lett. 2014 Dec 20;588(24):4597-603.
Drimenol, a sesquiterpene alcohol, and its derivatives display diverse bio-activities in nature. However, a Drimenol synthase gene has yet to be identified. We identified a new sesquiterpene synthase cDNA (VoTPS3) in valerian plant (Valeriana officinalis). Purification and NMR analyses of the VoTPS3-produced terpene, and characterization of the VoTPS3 enzyme confirmed that VoTPS3 synthesizes (-)-Drimenol. In feeding assays, possible reaction intermediates, farnesol and drimenyl diphosphate, could not be converted to Drimenol, suggesting that the intermediate remains tightly bound to VoTPS3 during catalysis. A mechanistic consideration of (-)-Drimenol synthesis suggests that Drimenol synthase is likely to use a protonation-initiated cyclization, which is rare for sesquiterpene synthases. VoTPS3 can be used to produce (-)-Drimenol, from which useful drimane-type terpenes can be synthesized.
Antifungal Effect of Polygodial on Botrytis cinerea, a Fungal Pathogen Affecting Table Grapes.[Pubmed:29077000]
Int J Mol Sci. 2017 Oct 27;18(11). pii: ijms18112251.
The antifungal activity of polygodial, a secondary metabolite extracted from Canelo, on mycelial growth of different Botrytis cinerea isolates has been evaluated. The results show that polygodial affects growth of normal and resistant isolates of B. cinerea with EC50 values ranging between 117 and 175 ppm. In addition, polygodial markedly decreases the germination of B. cinerea, i.e., after six hours of incubation the percentage of germination decreases from 92% (control) to 25% and 5% in the presence of 20 ppm and 80 ppm of polygodial, respectively. Morphological studies indicate that conidia treated with polygodial are smaller, with irregular membrane border, and a lot of cell debris, as compared to conidia in the control. The existence of polygodial-induced membrane damage was confirmed by SYTOX((R)) Green uptake assay. Gene expression studies confirm that the effect of polygodial on B. cinerea is mainly attributed to inhibition of germination and appears at early stages of B. cinerea development. On the other hand, Drimenol, a drimane with chemical structure quite similar to polygodial, inhibits the mycelial growth efficiently. Thus, both compounds inhibit mycelial growth by different mechanisms. The different antifungal activities of these compounds are discussed in terms of the electronic density on the double bond.
Effect of drimenol and synthetic derivatives on growth and germination of Botrytis cinerea: Evaluation of possible mechanism of action.[Pubmed:28911740]
Pestic Biochem Physiol. 2017 Sep;141:50-56.
The aim of this study was to determine the antifungal activity of Drimenol (1) and its synthetic derivatives, nordrimenone (2), drimenyl acetate (3), and drimenyl-epoxy-acetate (4), and to establish a possible mechanism of action for Drimenol. For that, the effect of each compound on mycelial growth of Botrytis cinerea was assessed. Our results showed that compounds 1, 2, 3 and 4 are able to affect Botrytis cinerea growth with EC50 values of 80, 92, 80 and 314ppm, respectively. These values suggest that the activity of these compounds is mainly determined by presence of the double bond between carbons 7 and 8 of the drimane ring. In addition, germination of B. cinerea in presence of 40 and 80ppm of Drimenol is reduced almost to a half of the control value. Finally, in order to elucidate a possible mechanism by which Drimenol is affecting B. cinerea, the determination of membrane integrity, reactive oxygen species production and gene expression studies of specific genes were performed.
Antifeedant effect of polygodial and drimenol derivatives against Spodoptera frugiperda and Epilachna paenulata and quantitative structure-activity analysis.[Pubmed:29316155]
Pest Manag Sci. 2018 Jul;74(7):1623-1629.
BACKGROUND: The antifeedant activity of 18 sesquiterpenoids of the drimane family (polygodial, Drimenol and derivatives) was investigated. RESULTS: Polygodial, drimanic and nordrimanic derivatives were found to exert antifeedant effects against two insect species, Spodoptera frugiperda and Epilachna paenulata, which are pests of agronomic interest, indicating that they have potential as biopesticide agents. Among the 18 compounds tested, the epoxynordrimane compound (11) and isonordrimenone (4) showed the highest activity [50% effective concentration (EC50 ) = 23.28 and 25.63 nmol cm(-)(2) , respectively, against S. frugiperda, and 50.50 and 59.00 nmol/cm(2) , respectively, against E. paenulata]. CONCLUSION: The results suggest that drimanic compounds have potential as new agents against S. frugiperda and E. paenulata. A quantitative structure-activity relationship (QSAR) analysis of the whole series, supported by electronic studies, suggested that drimanic compounds have structural features necessary for increasing antifeedant activity, namely a C-9 carbonyl group and an epoxide at C-8 and C-9. (c) 2018 Society of Chemical Industry.
Bioassay-Guided Isolation of Cytotoxic Isocryptoporic Acids from Cryptoporus volvatus.[Pubmed:27941653]
Molecules. 2016 Dec 8;21(12). pii: molecules21121692.
The present work constitutes a contribution to the phytochemical investigation of Cryptoporus volvatus aiming to search for effective cytotoxic constituents against tumor cell lines in vivo. Bioassay-guided separation of the ethylacetate extract of C. volvatus afforded four new isocryptoporic acid (ICA) derivatives, ICA-B trimethyl ester (1), ICA-E (2), ICA-E pentamethyl ester (3), and ICA-G (4), together with nine known cryptoporic acids. These isocryptoporic acids are isomers of the cryptoporic acids with Drimenol instead of albicanol as the terpenoid fragment; their structures were elucidated on the basis of spectroscopic evidences (UV, IR, HRMS, and NMR) and comparison with literature values. All isolates show certain cytotoxic activities against five tumor cell lines. Among them, compound 4 showed an comparable activity to that of the positive control cis-platin, while other compounds exhibited weak cytotoxic activities.


