Phenylacetic AcidCAS# 103-82-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 103-82-2 | SDF | Download SDF |
| PubChem ID | 999 | Appearance | Powder |
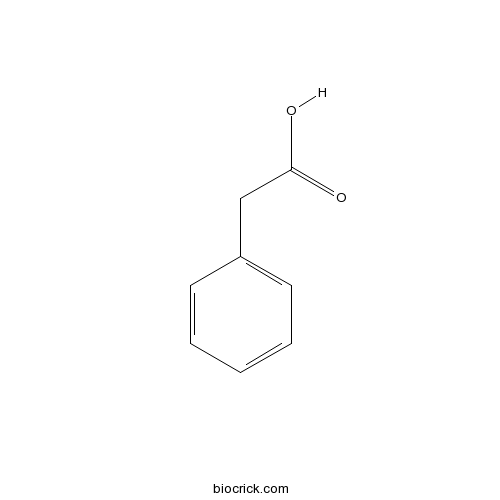
| Formula | C8H8O2 | M.Wt | 136 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-phenylacetic acid | ||
| SMILES | C1=CC=C(C=C1)CC(=O)O | ||
| Standard InChIKey | WLJVXDMOQOGPHL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H8O2/c9-8(10)6-7-4-2-1-3-5-7/h1-5H,6H2,(H,9,10) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Phenylacetic Acid Dilution Calculator

Phenylacetic Acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.3529 mL | 36.7647 mL | 73.5294 mL | 147.0588 mL | 183.8235 mL |
| 5 mM | 1.4706 mL | 7.3529 mL | 14.7059 mL | 29.4118 mL | 36.7647 mL |
| 10 mM | 0.7353 mL | 3.6765 mL | 7.3529 mL | 14.7059 mL | 18.3824 mL |
| 50 mM | 0.1471 mL | 0.7353 mL | 1.4706 mL | 2.9412 mL | 3.6765 mL |
| 100 mM | 0.0735 mL | 0.3676 mL | 0.7353 mL | 1.4706 mL | 1.8382 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- N-Methylbenzylamine
Catalog No.:BCN1790
CAS No.:103-67-3
- Scutebarbatine M
Catalog No.:BCN8327
CAS No.:960302-92-5
- Benzyl cinnamate
Catalog No.:BCN5042
CAS No.:103-41-3
- Ethyl cinnamate
Catalog No.:BCN5044
CAS No.:103-36-6
- Methyl cinnamate
Catalog No.:BCN5043
CAS No.:103-26-4
- Monobenzone
Catalog No.:BCC3818
CAS No.:103-16-2
- H-DL-Phg-OH
Catalog No.:BCC3317
CAS No.:103-01-5
- Trelagliptin succinate
Catalog No.:BCC2015
CAS No.:1029877-94-8
- INCB28060
Catalog No.:BCC3793
CAS No.:1029712-80-8
- Scutellaric acid
Catalog No.:BCN5843
CAS No.:102919-76-6
- MDL 73005EF hydrochloride
Catalog No.:BCC6636
CAS No.:102908-60-1
- Pexidartinib (PLX3397)
Catalog No.:BCC6405
CAS No.:1029044-16-3
- 4'-Methylacetanilide
Catalog No.:BCC8714
CAS No.:103-89-9
- Acetaminophen
Catalog No.:BCC5269
CAS No.:103-90-2
- Dehydroheliobuphthalmin
Catalog No.:BCN5844
CAS No.:103001-05-4
- Alterlactone
Catalog No.:BCN7261
CAS No.:1030376-89-6
- MK-4305
Catalog No.:BCC1760
CAS No.:1030377-33-3
- CTCE 9908
Catalog No.:BCC6366
CAS No.:1030384-98-5
- Ethyl 3-(pyridin-2-ylamino)propanoate
Catalog No.:BCC8973
CAS No.:103041-38-9
- Daptomycin
Catalog No.:BCC1057
CAS No.:103060-53-3
- MK-8245
Catalog No.:BCC2299
CAS No.:1030612-90-8
- AS 2034178
Catalog No.:BCC7996
CAS No.:1030846-42-4
- Bakuchiol
Catalog No.:BCN5845
CAS No.:10309-37-2
- 2-Amino-6-chloropurine
Catalog No.:BCC8540
CAS No.:10310-21-1
Directed Iridium-Catalyzed Hydrogen Isotope Exchange Reactions of Phenylacetic Acid Esters and Amides.[Pubmed:30932249]
Chemistry. 2019 Apr 1.
For the first time, a catalytic protocol for highly selective hydrogen isotope exchange (HIE) of Phenylacetic Acid esters and amides under very mild reaction conditions is reported. Using a homogeneous iridium catalyst supported by a bidentate phosphine-imidazolin-2-imine P,N ligand, the HIE reaction on a series of Phenylacetic Acid derivatives proceeds with high yields, high selectivity and with deuterium incorporation up to 99%. The method is fully adaptable to the specific requirements of tritium chemistry, and its effectiveness was demonstrated by direct tritium labeling of the fungicide benalaxyl and the drug camylofine. Further insights into the mechanism of the HIE reaction with catalyst 1 have been provided utilizing DFT calculation, NMR-studies and X-ray diffraction analysis.
Shunting Phenylacetic Acid Catabolism for Tropone Biosynthesis.[Pubmed:30861343]
ACS Synth Biol. 2019 Mar 26.
Tropone is a seven-membered ring nonbenzenoid aromatic compound. It is the core structure of tropolonoids, which have various biological activities. In this study, a hybrid tropone biosynthetic pathway was designed by connecting Phenylacetic Acid (PAA) degradation with its biosynthesis and reconstituted in Escherichia coli. To simplify pathway construction and optimization, the use of E. coli endogenous genes was maximized and only three exogenous genes were employed. The entire pathway was divided into four modules: the endogenous shikimate pathway module, the hybrid PAA biosynthetic module, the endogenous PAA catabolic module and the heterogeneous tropone biosynthetic module. Efficiency of the PAA catabolic module was enhanced using PAA consumption rate as the indicator. Then, a single point mutation was introduced to inactivate the ALDH domain of PaaZ and the carbon flow was redirected toward tropone synthesis. Assembly of the full pathway led to de novo tropone production with the best titer of 65.2 +/- 1.4 mg/L in shake flask experiment. This study provides a potential alternative for sustainable production of tropone and its derivatives.
Metabolic Engineering of Escherichia coli for para-Amino-Phenylethanol and para-Amino-Phenylacetic Acid Biosynthesis.[Pubmed:30662895]
Front Bioeng Biotechnol. 2019 Jan 4;6:201.
Aromatic amines are an important class of chemicals which are used as building blocks for the synthesis of polymers and pharmaceuticals. In this study we establish a de novo pathway for the biosynthesis of the aromatic amines para-amino-phenylethanol (PAPE) and para-amino-Phenylacetic Acid (4-APA) in Escherichia coli. We combined a synthetic para-amino-l-phenylalanine pathway with the fungal Ehrlich pathway. Therefore, we overexpressed the heterologous genes encoding 4-amino-4-deoxychorismate synthase (pabAB from Corynebacterium glutamicum), 4-amino-4-deoxychorismate mutase and 4-amino-4-deoxyprephenate dehydrogenase (papB and papC from Streptomyces venezuelae) and ThDP-dependent keto-acid decarboxylase (aro10 from Saccharomyces cerevisiae) in E. coli. The resulting para-amino-phenylacetaldehyde either was reduced to PAPE or oxidized to 4-APA. The wild type strain E. coli LJ110 with a plasmid carrying these four genes produced (in shake flask cultures) 11 +/- 1.5 mg l(-1) of PAPE from glucose (4.5 g l(-1)). By the additional cloning and expression of feaB (phenylacetaldehyde dehydrogenase from E. coli) 36 +/- 5 mg l(-1) of 4-APA were obtained from 4.5 g l(-1) glucose. Competing reactions, such as the genes for aminotransferases (aspC and tyrB) or for biosynthesis of L-phenylalanine and L-tyrosine (pheA, tyrA) and for the regulator TyrR were removed. Additionally, the E. coli genes aroFBL were cloned and expressed from a second plasmid. The best producer strains of E. coli showed improved formation of PAPE and 4-APA, respectively. Plasmid-borne expression of an aldehyde reductase (yahK from E. coli) gave best values for PAPE production, whereas feaB-overexpression led to best values for 4-APA. In fed-batch cultivation, the best producer strains achieved 2.5 +/- 0.15 g l(-1) of PAPE from glucose (11% C mol mol-1 glucose) and 3.4 +/- 0.3 g l(-1) of 4-APA (17% C mol mol(-1) glucose), respectively which are the highest values for recombinant strains reported so far.
An Historical Review of Phenylacetic Acid.[Pubmed:30649529]
Plant Cell Physiol. 2019 Jan 15. pii: 5289543.
Plant hormone biology is an ever-evolving field and as such, novel avenues of research must always be sought. Technological and theoretical advancement can also allow for previously dismissed research to yield equally interesting insights into processes now that they are better understood. The auxin PAA is an excellent example of this. PAA is a plant auxin that also possesses substantial anti-microbial activity. It has a broad distribution and has been studied in bacteria, fungi, algae and land plants. Research on this compound in plants was prominent in the 1980s, where its bioactivity and broad distribution were frequently examined. Unfortunately, due to the strong interest in the quintessential auxin, IAA, research on PAA quickly petered out. Recently, several groups have resumed investigations on this hormone in plants, yet, little is known about PAA biology and its physiological role is unclear. PAA biosynthesis from the amino acid Phe invites direct comparisons with previously studied IAA biosynthesis pathways, and recent work has shown that PAA metabolism and signaling appears to be similar to that of IAA. However, given the large gap between previous work and recent investigations, a historical review of this auxin is required to renew our understanding of PAA. Here, previous work on PAA is reassessed in light of recent research in plants and serves as a synthesis of current knowledge on PAA biology.
Cell-Based Biosensor with Dual Signal Outputs for Simultaneous Quantification of Phenylacetic Acid and Phenylethylamine.[Pubmed:30418753]
ACS Synth Biol. 2018 Nov 15.
Despite the importance of 2-Phenylacetic Acid, a plant hormone in the endogenous auxin family, its biosynthesis pathway has yet to be elucidated. In this study, we developed a novel whole-cell biosensor for the simultaneous quantification of 2-Phenylacetic Acid (PA) and 2-phenylethylamine (PEA) through the regulation of bacterial catabolism of aromatic compounds. We used the PA regulon to enable the recognition of PA and PEA. Differentiation of PEA from PA involves the incorporation of the FeaR regulon within the same whole-cell biosensor to report the presence of aromatic amines. The proposed system is highly sensitive to PA as well as PEA.


