ParecoxibCAS# 198470-84-7 |
- Amyloid β-Protein (1-15)
Catalog No.:BCC1003
CAS No.:183745-81-5
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 198470-84-7 | SDF | Download SDF |
| PubChem ID | 119828 | Appearance | Powder |
| Formula | C19H18N2O4S | M.Wt | 370.42 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | SC 69124 | ||
| Solubility | DMSO : ≥ 50 mg/mL (134.98 mM) *"≥" means soluble, but saturation unknown. | ||
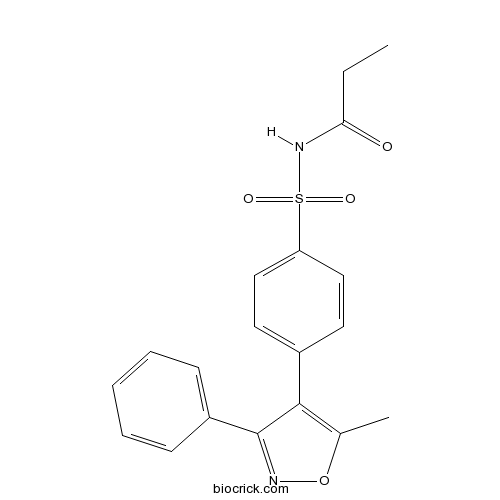
| Chemical Name | N-[4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)phenyl]sulfonylpropanamide | ||
| SMILES | CCC(=O)NS(=O)(=O)C1=CC=C(C=C1)C2=C(ON=C2C3=CC=CC=C3)C | ||
| Standard InChIKey | TZRHLKRLEZJVIJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H18N2O4S/c1-3-17(22)21-26(23,24)16-11-9-14(10-12-16)18-13(2)25-20-19(18)15-7-5-4-6-8-15/h4-12H,3H2,1-2H3,(H,21,22) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Parecoxib is a potent and selective COX-2 inhibitor.
IC50 value:
Target: COX-2
in vitro: The prodrug Parecoxib as well as its active metabolite val have a specific affinity to the cannabinoid (CB) receptor measured in CB1-expressing HEK 293 cells and rat brain tissue [1].
in vivo: Adult male Sprague-Dawley rats were administered parecoxib (10 or 30 mg kg(-1), IP) or isotonic saline twice a day starting 24 h after middle cerebral artery occlusion (MCAO) for three consecutive days [2]. The selective COX-2 inhibitor parecoxib was delivered 20 min before or 20 min after the incision by intraperitoneal injection. Pretreatment with parecoxib markedly attenuated the pain hypersensitivity induced by incision [3]. References: | |||||

Parecoxib Dilution Calculator

Parecoxib Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6996 mL | 13.4982 mL | 26.9964 mL | 53.9928 mL | 67.491 mL |
| 5 mM | 0.5399 mL | 2.6996 mL | 5.3993 mL | 10.7986 mL | 13.4982 mL |
| 10 mM | 0.27 mL | 1.3498 mL | 2.6996 mL | 5.3993 mL | 6.7491 mL |
| 50 mM | 0.054 mL | 0.27 mL | 0.5399 mL | 1.0799 mL | 1.3498 mL |
| 100 mM | 0.027 mL | 0.135 mL | 0.27 mL | 0.5399 mL | 0.6749 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Parecoxib is a potent and selective COX-2 inhibitor.
- Boc-D-Pen(pMeBzl)-OH.DCHA
Catalog No.:BCC3308
CAS No.:198470-36-9
- LY 367385
Catalog No.:BCC6983
CAS No.:198419-91-9
- Erythrodiol 3-palmitate
Catalog No.:BCN4869
CAS No.:19833-13-7
- Myricetin 3-O-beta-D-glucopyranoside
Catalog No.:BCN8144
CAS No.:19833-12-6
- Medicagol
Catalog No.:BCN8430
CAS No.:1983-72-8
- Gap 27
Catalog No.:BCC1033
CAS No.:198284-64-9
- Triptobenzene K
Catalog No.:BCN8055
CAS No.:198129-88-3
- GW311616
Catalog No.:BCC5393
CAS No.:198062-54-3
- AM 404
Catalog No.:BCC6945
CAS No.:198022-70-7
- GW311616 hydrochloride
Catalog No.:BCC5394
CAS No.:197890-44-1
- Stachartin E
Catalog No.:BCN6970
CAS No.:1978388-58-7
- Stachartin D
Catalog No.:BCN6971
CAS No.:1978388-57-6
- Parecoxib Sodium
Catalog No.:BCC4248
CAS No.:198470-85-8
- Boc-Pen(pMeBzl)-OH.DCHA
Catalog No.:BCC2623
CAS No.:198474-61-2
- Bazedoxifene HCl
Catalog No.:BCC4492
CAS No.:198480-56-7
- Bazedoxifene
Catalog No.:BCC1411
CAS No.:198481-32-2
- Bazedoxifene acetate
Catalog No.:BCC1412
CAS No.:198481-33-3
- 9,10-Anthracenedione
Catalog No.:BCN3469
CAS No.:19852-76-7
- Fmoc-Asparaginol(Trt)
Catalog No.:BCC3042
CAS No.:198543-08-7
- Fmoc-HoTyr-OH.DCHA
Catalog No.:BCC3246
CAS No.:198560-10-0
- Fmoc-Ser(tBu)-ol
Catalog No.:BCC2578
CAS No.:198561-87-4
- Tranylcypromine hydrochloride
Catalog No.:BCC7791
CAS No.:1986-47-6
- Alisol A 23-acetate
Catalog No.:BCN3457
CAS No.:19865-75-9
- Alisol B acetate
Catalog No.:BCN2304
CAS No.:19865-76-0
Parecoxib reduced ventilation induced lung injury in acute respiratory distress syndrome.[Pubmed:28356130]
BMC Pharmacol Toxicol. 2017 Mar 29;18(1):25.
BACKGROUND: Cyclooxygenase-2 (COX-2) contributes to ventilation induced lung injury (VILI) and acute respiratory distress syndrome (ARDS). The objective of present study was to observe the therapeutic effect of Parecoxib on VILI in ARDS. METHODS: In this parallel controlled study performed at Harbin Medical University, China between January 2016 and March 2016, 24 rats were randomly allocated into sham group (S), volume ventilation group/ARDS (VA), Parecoxib/volume ventilation group/ARDS (PVA). Rats in the S group only received anesthesia; rats in the VA and PVA group received intravenous injection of endotoxin to induce ARDS, and then received ventilation. Rats in the VA and PVA groups were treated with intravenous injection of saline or Parecoxib. The ratio of arterial oxygen pressure to fractional inspired oxygen (PaO2/FiO2), the wet to dry weight ratio of lung tissue, inflammatory factors in serum and bronchoalveolar lavage fluid (BALF), and histopathologic analyses of lung tissue were examined. In addition, survival was calculated at 24 h after VILI. RESULTS: Compared to the VA group, in the PVA group, PaO2/FiO2 was significantly increased; lung tissue wet to dry weight ratio; macrophage and neutrophil counts, total protein and neutrophil elastase levels in BALF; tumor necrosis factor-alpha, interleukin-1beta, and prostaglandin E2 levels in BALF and serum; and myeloperoxidase (MPO) activity, malondialdehyde levels, and Bax and COX-2 protein levels in lung tissue were significantly decreased, while Bcl-2 protein levels were significantly increased. Lung histopathogical changes and apoptosis were reduced by parecpxib in the PVA group. Survival was increased in the PVA group. CONCLUSIONS: Parecoxib improves gas exchange and epithelial permeability, decreases edema, reduces local and systemic inflammation, ameliorates lung injury and apoptosis, and increases survival in a rat model of VILI.
Parecoxib sodium pretreatment reduces myoclonus after etomidate: A prospective, double-blind, randomized clinical trial.[Pubmed:28291508]
Int J Clin Pharmacol Ther. 2017 Jul;55(7):601-605.
OBJECTIVE: Myoclonus induced by etomidate during induction of general anesthesia is a common phenomenon. This prospective, randomized, saline-controlled clinical study was performed to evaluate the effect of Parecoxib sodium pretreatment on the incidence and severity of etomidate-induced myoclonus. METHODS: 60 patients, American Society of Anesthesiologists (ASA) physical status I or II, aged 20 to 60 years, who were scheduled to undergo elective laparoscopic cholecystectomy under general anesthesia, were allocated randomly into one of two groups to receive Parecoxib sodium 40 mg intravenous (group P, n = 30) or the same volume of saline (group S, n = 30) 30 minutes before administration of etomidate (0.3 mg/kg). Myoclonus was assessed on a scale of 0 - 3. Postoperative side effects were recorded. RESULTS: The two groups were comparable with regard to baseline characteristics. The incidence of myoclonus was significantly lower in the Parecoxib sodium group (11/30; 37%) than in the saline group (21/30; 70%) (p < 0.05). The severity of myoclonic movements was also significantly reduced by Parecoxib sodium (p < 0.05). There were no significant differences between the two groups with respect to postoperative side effects. CONCLUSIONS: Pretreatment with intravenous injection of Parecoxib sodium 40 mg significantly reduced the incidence and severity of etomidate-induced myoclonus without significant side effects..
Parecoxib Provides Analgesic and Opioid-Sparing Effects Following Major Orthopedic Surgery: A Subset Analysis of a Randomized, Placebo-Controlled Clinical Trial.[Pubmed:28255955]
Pain Ther. 2017 Jun;6(1):61-72.
INTRODUCTION: Orthopedic surgeries are among the most common and most painful surgeries performed. A multimodal analgesic approach is recommended to reduce opioid consumption, provide effective pain relief, and improve outcomes following surgery. This study examined the efficacy and opioid-sparing effects of Parecoxib following major orthopedic surgery. METHODS: This subset analysis of a large, multicenter, randomized, double-blind, placebo-controlled study of Parecoxib examined treatment effects on postoperative pain severity, pain interference with function, opioid consumption, occurrence of opioid-related symptoms, safety, and patient satisfaction following major orthopedic surgery. RESULTS: Pain scores were significantly lower in the Parecoxib group (n = 142) compared with placebo (n = 139) on day 2 (-22%; p < 0.001) and day 3 (-17%; p = 0.004). Pain interference with function scores were also significantly lower in the Parecoxib group on day 2 (-32%; p < 0.001) and day 3 (-27%; p = 0.003) relative to placebo. Additionally, significantly less supplemental morphine was required in the Parecoxib group relative to placebo through 24 h (-28%; p = 0.008) and 48 h (-33%; p < 0.001). Patients in the Parecoxib group had a reduced risk of experiencing opioid-related symptoms including fatigue, drowsiness, inability to concentrate, confusion, nausea, constipation, and confusion on day 2 and/or day 3. Finally, more patients receiving Parecoxib (42%) rated treatment as "excellent" compared to those receiving placebo (21%). CONCLUSIONS: These findings support the use of Parecoxib for the management of pain following major orthopedic surgery.
Effects of parecoxib on postoperative pain and opioid-related symptoms following gynecologic surgery.[Pubmed:27932894]
J Pain Res. 2016 Nov 25;9:1101-1107.
OBJECTIVE: To examine the analgesic and opioid-sparing effects of Parecoxib following major gynecologic surgery. METHODS: This is a large subset analysis of patients from a multicenter, randomized, double-blind, placebo-controlled study of Parecoxib/valdecoxib (PAR/VAL) for postoperative pain. Pain severity, pain interference with function, opioid use, occurrence of opioid-related symptoms, and Patient/Physician Global Evaluation of Study Medication were compared between placebo and PAR/VAL treatment groups in the days following surgery. RESULTS: Pain scores were reduced in the PAR/VAL group (n=98), relative to placebo (n=97), on Day 2 (-21%, P<0.001) and Day 3 (-23%, P=0.004). Pain interference with function scores were also significantly lower in the PAR/VAL group, compared with placebo, on Day 2 (-29%, P<0.001) and Day 3 (-28%, P=0.013). Consumption of supplemental morphine was significantly lower in the PAR/VAL group relative to placebo at 24 hours (-37%, P=0.010) and trended lower at 48 (-28%) and 72 hours (-26%). Patients in the PAR/VAL group also had a reduced risk of experiencing specific opioid-related symptoms, including "inability to concentrate" (relative risk =0.53) and "nausea" (relative risk =0.60) on Day 2. Both Patient and Physician Global Evaluation of Study Medication scores were better in the PAR/VAL group than in the placebo group. CONCLUSION: The current study adds support for the use of Parecoxib in patients following major gynecologic surgery.


