PalustrolCAS# 5986-49-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 5986-49-2 | SDF | Download SDF |
| PubChem ID | 110745 | Appearance | Powder |
| Formula | C15H26O | M.Wt | 222.4 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1,1,4,7-tetramethyl-2,3,4,5,6,7,7a,7b-octahydro-1aH-cyclopropa[h]azulen-4a-ol | ||
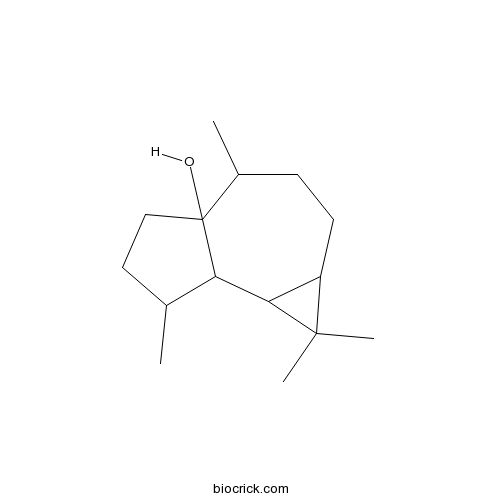
| SMILES | CC1CCC2(C1C3C(C3(C)C)CCC2C)O | ||
| Standard InChIKey | QWRTXOOFEHOROQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H26O/c1-9-7-8-15(16)10(2)5-6-11-13(12(9)15)14(11,3)4/h9-13,16H,5-8H2,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Palustrol exhibits acaricidal, insecticidal, ‘pesticidal’ and/or arthropod repellent properties, may be useful sources of chemicals for the control of arthropods of medical, veterinary or agricultural importance. |
| Targets | Antifection |

Palustrol Dilution Calculator

Palustrol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.4964 mL | 22.482 mL | 44.964 mL | 89.9281 mL | 112.4101 mL |
| 5 mM | 0.8993 mL | 4.4964 mL | 8.9928 mL | 17.9856 mL | 22.482 mL |
| 10 mM | 0.4496 mL | 2.2482 mL | 4.4964 mL | 8.9928 mL | 11.241 mL |
| 50 mM | 0.0899 mL | 0.4496 mL | 0.8993 mL | 1.7986 mL | 2.2482 mL |
| 100 mM | 0.045 mL | 0.2248 mL | 0.4496 mL | 0.8993 mL | 1.1241 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Synephrine HCl
Catalog No.:BCC4359
CAS No.:5985-28-4
- Ajmalimine
Catalog No.:BCN3420
CAS No.:59846-31-0
- Tenoxicam
Catalog No.:BCC4733
CAS No.:59804-37-4
- UK 14,304
Catalog No.:BCC5226
CAS No.:59803-98-4
- Vindolinine
Catalog No.:BCN7822
CAS No.:5980-02-9
- Cyclosporin C
Catalog No.:BCC8160
CAS No.:59787-61-0
- 6beta-Angeloyloxy-1beta,10beta-epoxy-9-oxofuranoeremophilane
Catalog No.:BCN7600
CAS No.:59780-08-4
- Boc-Asp(OMe)-OH.DCHA
Catalog No.:BCC3367
CAS No.:59768-74-0
- Cudraflavanone B
Catalog No.:BCN3446
CAS No.:597542-74-0
- 1beta,10beta-Epoxy-6beta-isobutyryloxy-9-oxofuranoeremophilane
Catalog No.:BCN7601
CAS No.:59742-11-9
- beta-Amyrin palmitate
Catalog No.:BCN4099
CAS No.:5973-06-8
- Citalopram hydrobromide
Catalog No.:BCC7063
CAS No.:59729-32-7
- Patchouli alcohol
Catalog No.:BCN4966
CAS No.:5986-55-0
- Cyclosporin A
Catalog No.:BCC4773
CAS No.:59865-13-3
- Oxybuprocaine HCl
Catalog No.:BCC4691
CAS No.:5987-82-6
- Glabridin
Catalog No.:BCN1221
CAS No.:59870-68-7
- L-Dihydroorotic acid
Catalog No.:BCC9007
CAS No.:5988-19-2
- N-Formyl-Met-Leu-Phe
Catalog No.:BCC7205
CAS No.:59880-97-6
- ent-7alpha,9-Dihydroxy-15-oxokaur-16-en-19,6bet-olide
Catalog No.:BCN7401
CAS No.:59885-89-1
- 7-hydroxy-4-benzopyrone
Catalog No.:BCC9209
CAS No.:59887-89-7
- Loliolid
Catalog No.:BCN3655
CAS No.:5989-02-6
- Mesopsin
Catalog No.:BCN8050
CAS No.:5989-16-2
- Medicagenic acid
Catalog No.:BCN3893
CAS No.:599-07-5
- 3,3'-Sulfonyldianiline
Catalog No.:BCC8595
CAS No.:599-61-1
Birch (Betula spp.) leaves adsorb and re-release volatiles specific to neighbouring plants--a mechanism for associational herbivore resistance?[Pubmed:20298484]
New Phytol. 2010 May;186(3):722-32.
Plant-emitted semi-volatile compounds have low vaporization rates at 20-25 degrees C and may therefore persist on surfaces such as plant foliage. The passive adsorption of arthropod-repellent semi-volatiles to neighbouring foliage could convey associational resistance, whereby a plant's neighbours reduce damage caused by herbivores. We found that birch (Betula spp.) leaves adsorb and re-release the specific arthropod-repelling C(15) semi-volatiles ledene, ledol and Palustrol produced by Rhododendron tomentosum when grown in mixed association in a field setup. In a natural habitat, a higher concentration of ledene was released from birches neighbouring R. tomentosum than from birches situated > 5 m from R. tomentosum. Emission of alpha-humulene, a sesquiterpene synthesized by both Betula pendula and R. tomentosum, was also increased in R. tomentosum-neighbouring B. pendula. In assessments for associational resistance, we found that the polyphagous green leaf weevils (Polydrusus flavipes) and autumnal moth (Epirrita autumnata) larvae both preferred B. pendula to R. tomentosum. P. flavipes also preferred birch leaves not exposed to R. tomentosum to leaves from mixed associations. In the field, a reduction in Euceraphis betulae aphid density occurred in mixed associations. Our results suggest that plant/tree species may be protected by semi-volatile compounds emitted by a more herbivore-resistant heterospecific neighbour.
Evaluation of extracts and oils of tick-repellent plants from Sweden.[Pubmed:16336298]
Med Vet Entomol. 2005 Dec;19(4):345-52.
Abstract. Leaves of Myrica gale Linnaeus (Myricaceae), Rhododendron tomentosum (Stokes) H. Harmaja (formerly Ledum palustre Linnaeus: Ericaceae) and Artemisia absinthium Linnaeus (Asteraceae) were extracted with organic solvents of different polarities and the essential oils of leaves were obtained by steam distillation. The extracts or oils were tested in the laboratory for repellency against host-seeking nymphs of Ixodes ricinus Linnaeus (Acari: Ixodidae). Rhododendron tomentosum oil, 10%, diluted in acetone, exhibited 95% repellency; R. tomentosum and A. absinthium extracts in ethyl acetate, > 70% repellency; A. absinthium extract in hexane, approximately 62% repellency; and M. gale oil, 10%, approximately 50% repellency on I. ricinus nymphs. Compounds in the leaf extracts or in the oils were collected by solid phase microextraction (SPME) and identified by gas chromatography-mass spectrometry (GC-MS) and/or MS. Characteristic volatiles detected from oil or extract of M. gale were the monoterpenes 1,8-cineole, alpha-terpineol, 4-terpineol and thujenol; and of R. tomentosum myrcene and Palustrol. Characteristic volatiles from leaf extracts of A. absinthium were sabinene, oxygenated monoterpenes, e.g. thujenol and linalool, and geranyl acetate. Each plant species synthesized numerous volatiles known to exhibit acaricidal, insecticidal, 'pesticidal' and/or arthropod repellent properties. These plants may be useful sources of chemicals for the control of arthropods of medical, veterinary or agricultural importance.


