(±)-PPCC oxalateCAS# 932736-91-9 |
- BMS-708163 (Avagacestat)
Catalog No.:BCC2104
CAS No.:1146699-66-2
- DAPT (GSI-IX)
Catalog No.:BCC3618
CAS No.:208255-80-5
- YO-01027 (Dibenzazepine, DBZ)
Catalog No.:BCC2100
CAS No.:209984-56-5
- MK-0752
Catalog No.:BCC2090
CAS No.:471905-41-6
- MRK 560
Catalog No.:BCC2345
CAS No.:677772-84-8
- PF-03084014
Catalog No.:BCC1848
CAS No.:865773-15-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 932736-91-9 | SDF | Download SDF |
| PubChem ID | 90488889 | Appearance | Powder |
| Formula | C52H62N2O14 | M.Wt | 939.1 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 10 mM in ethanol | ||
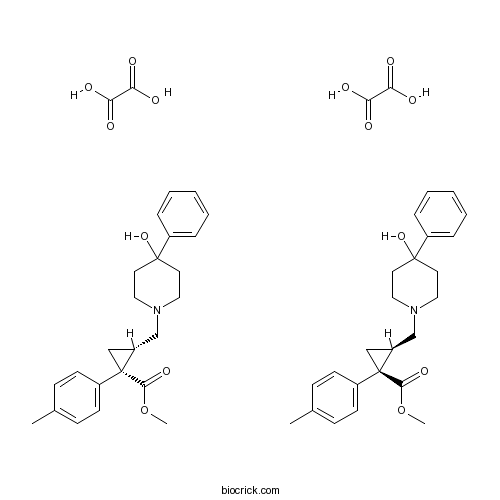
| Chemical Name | methyl (1S,2R)-2-[(4-hydroxy-4-phenylpiperidin-1-yl)methyl]-1-(4-methylphenyl)cyclopropane-1-carboxylate;methyl (1R,2S)-2-[(4-hydroxy-4-phenylpiperidin-1-yl)methyl]-1-(4-methylphenyl)cyclopropane-1-carboxylate;oxalic acid | ||
| SMILES | CC1=CC=C(C=C1)C2(CC2CN3CCC(CC3)(C4=CC=CC=C4)O)C(=O)OC.CC1=CC=C(C=C1)C2(CC2CN3CCC(CC3)(C4=CC=CC=C4)O)C(=O)OC.C(=O)(C(=O)O)O.C(=O)(C(=O)O)O | ||
| Standard InChIKey | IGJDSEGFEVKASG-KLELIYFTSA-N | ||
| Standard InChI | InChI=1S/2C24H29NO3.2C2H2O4/c2*1-18-8-10-20(11-9-18)24(22(26)28-2)16-21(24)17-25-14-12-23(27,13-15-25)19-6-4-3-5-7-19;2*3-1(4)2(5)6/h2*3-11,21,27H,12-17H2,1-2H3;2*(H,3,4)(H,5,6)/t2*21-,24+;;/m10../s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective sigma (σ) receptor ligand. Displays high affinity for σ1; also binds at σ2 sites (Ki = 1.5 nM and 50.8 nM respectively). Selective over a range of receptor types including dopaminergic and muscarinic receptors, DAT and SERT. |

(±)-PPCC oxalate Dilution Calculator

(±)-PPCC oxalate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.0648 mL | 5.3242 mL | 10.6485 mL | 21.297 mL | 26.6212 mL |
| 5 mM | 0.213 mL | 1.0648 mL | 2.1297 mL | 4.2594 mL | 5.3242 mL |
| 10 mM | 0.1065 mL | 0.5324 mL | 1.0648 mL | 2.1297 mL | 2.6621 mL |
| 50 mM | 0.0213 mL | 0.1065 mL | 0.213 mL | 0.4259 mL | 0.5324 mL |
| 100 mM | 0.0106 mL | 0.0532 mL | 0.1065 mL | 0.213 mL | 0.2662 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- CDDO-EA
Catalog No.:BCC5282
CAS No.:932730-51-3
- Boc-β-iodo-Ala-OMe
Catalog No.:BCC3052
CAS No.:93267-04-0
- Cistanoside A
Catalog No.:BCN2668
CAS No.:93236-42-1
- 5-Aminouracil
Catalog No.:BCC8737
CAS No.:932-52-5
- 2-Hydroxybenzylamine
Catalog No.:BCN1803
CAS No.:932-30-9
- PPQ-102
Catalog No.:BCC5248
CAS No.:931706-15-9
- IOX2(Glycine)
Catalog No.:BCC2229
CAS No.:931398-72-0
- Tacalcitol monohydrate
Catalog No.:BCC1976
CAS No.:93129-94-3
- (R,E)-Deca-2-ene-4,6-diyne-1,8-diol
Catalog No.:BCN4476
CAS No.:931116-24-4
- (S,E)-Deca-2,9-diene-4,6-diyne-1,8-diol
Catalog No.:BCN1305
CAS No.:931114-98-6
- Ciprofloxacin hydrochloride
Catalog No.:BCC8915
CAS No.:93107-08-5
- Enrofloxacin
Catalog No.:BCC4657
CAS No.:93106-60-6
- Iodoacetyl-LC-Biotin
Catalog No.:BCC3584
CAS No.:93285-75-7
- Monomethyl lithospermate
Catalog No.:BCN8124
CAS No.:933054-33-2
- 20-Hydroxy-3-oxo-28-lupanoic acid
Catalog No.:BCN4478
CAS No.:93372-87-3
- (+)-Taddol
Catalog No.:BCC8378
CAS No.:93379-49-8
- (S)-(-)-Atenolol
Catalog No.:BCC6633
CAS No.:93379-54-5
- 8-Epicrepiside E
Catalog No.:BCN7098
CAS No.:93395-30-3
- 8beta-(2-Hydroxy-2-methyl-3-oxobutyryloxy)glucozaluzanin C
Catalog No.:BCN6682
CAS No.:93395-31-4
- 2-Aminobenzimidazole
Catalog No.:BCC8547
CAS No.:934-32-7
- 2-Benzothiazolol
Catalog No.:BCC8557
CAS No.:934-34-9
- Chamaechromone
Catalog No.:BCN3718
CAS No.:93413-00-4
- Desvenlafaxine
Catalog No.:BCC5038
CAS No.:93413-62-8
- Venlafaxine
Catalog No.:BCC9190
CAS No.:93413-69-5
Anti-amnesic properties of (+/-)-PPCC, a novel sigma receptor ligand, on cognitive dysfunction induced by selective cholinergic lesion in rats.[Pubmed:19245662]
J Neurochem. 2009 May;109(3):744-54.
Previous studies have reported that selective sigma-1 agonists may improve cognitive abilities in experimental animals possibly via a cholinergic mechanism. However, the issue of a direct action on to sigma-1 receptors in memory-related brain areas has been much less investigated. The newly synthetised compound methyl(1R,2S/1S,2R)-2-[4-hydroxy-4-phenylpiperidin-1-yl)methyl]-1-(4-methylphenyl ) cyclopropanecarboxylate [(+/-)-PPCC] has recently been shown to possess high affinity for the sigma-1 receptor where it specifically acts as an agonist. Here, the functional effects of (+/-)-PPCC were investigated in rat models of mild or severe cognitive dysfunction based on a sub-total (
A new sigma ligand, (+/-)-PPCC, antagonizes kappa opioid receptor-mediated antinociceptive effect.[Pubmed:18261749]
Life Sci. 2008 Mar 12;82(11-12):549-53.
The compound (1R,2S/1S,2R)-2-[4-hydroxy-4-phenylpiperidin-1-yl)methyl]-1-(4-methylphenyl) cyclopropanecarboxylate [(+/-)-PPCC] is a ligand with high affinity for sigma (sigma) sites of which the selectivity towards several other receptor systems has been demonstrated. Given the existence of a relationship between the sigma system and the kappa opioid (KOP)-mediated analgesia, to characterize the pharmacological properties of (+/-)-PPCC we analyzed its influence on the analgesic effect of the systemic injected kappa agonist (-)-U-50,488H comparing the effects with those shown by (+)-pentazocine and BD1047. The results demonstrate that the systemic administration of (+/-)-PPCC (1 mg/kg s.c.) does not modify basal tail-flick latency. Pre-treatment with (+/-)-PPCC, at the same dose, significantly decreased the antinociceptive effect of (-)-U-50,488H, analogously to the sigma compounds used. This study confirms that (+/-)-PPCC plays the role of sigma agonist in this model and strengthens the hypothesis of the sigma receptor modulatory role on KOP-mediated analgesia.
Novel sigma receptor ligands: synthesis and biological profile.[Pubmed:17328523]
J Med Chem. 2007 Mar 8;50(5):951-61.
The aim of the present study was to investigate the biological profile of new substituted 1-phenyl-2-cyclopropylmethylamines. High affinity for both sigma subtypes was achieved when 4-phenylpiperidin-4-ol (4a-e) and 4-benzylpiperidine moieties were present (5a-e). (1R,2S/1S,2R)-2-[4-Hydroxy-4-phenylpiperidin-1-yl)methyl]-1-(4-methylphenyl)cyclo propanecarboxylate (4b) showed high affinity for the sigma1 sites (Ki = 1.5 nM) and the most favorable sigma1/sigma2 selectivity (Ki(sigma2)/Ki(sigma1) = 33.9). Binding affinity studies showed that 4b binding on N-methyl-d-aspartate (NMDA), dopaminergic (D1, D2, D3), muscarinic, histaminergic H1, adrenergic (alpha1, alpha2), serotoninergic (5-HT2A, 5-HT2C, 5-HT3, 5-HT4, 5-HT6), DA (DAT), and 5-HT (SERT) transporters was not significant. Interestingly, sigma ligands differently induced the expression of tissue transglutaminase (TG-2) in primary astroglial cell cultures. We suggest that 4b may act as a sigma1/sigma2 agonist and that the sigma ligands may modulate TG-2 differently.


