Cistanoside ACAS# 93236-42-1 |
Quality Control & MSDS
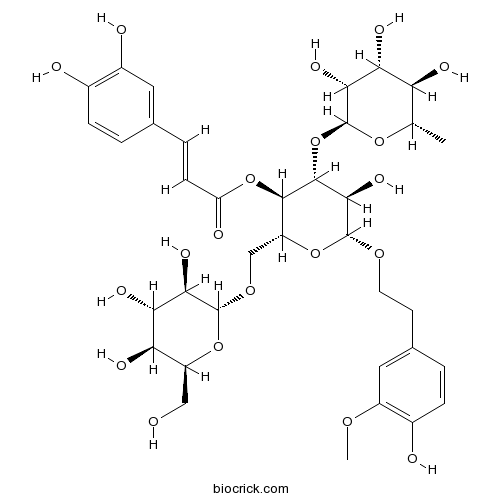
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 93236-42-1 | SDF | Download SDF |
| PubChem ID | 6450499 | Appearance | Powder |
| Formula | C36H48O20 | M.Wt | 800.75 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(2R,3R,4R,5R,6R)-5-hydroxy-6-[2-(4-hydroxy-3-methoxyphenyl)ethoxy]-2-[[(2R,3R,4S,5S,6S)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxymethyl]-4-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-3-yl] (E)-3-(3,4-dihydroxyphenyl)prop-2-enoate | ||
| SMILES | CC1C(C(C(C(O1)OC2C(C(OC(C2OC(=O)C=CC3=CC(=C(C=C3)O)O)COC4C(C(C(C(O4)CO)O)O)O)OCCC5=CC(=C(C=C5)O)OC)O)O)O)O | ||
| Standard InChIKey | LOGNFAUMIGACHZ-SCWCVTEDSA-N | ||
| Standard InChI | InChI=1S/C36H48O20/c1-15-25(42)27(44)30(47)36(52-15)56-33-31(48)35(50-10-9-17-4-7-19(39)21(12-17)49-2)54-23(14-51-34-29(46)28(45)26(43)22(13-37)53-34)32(33)55-24(41)8-5-16-3-6-18(38)20(40)11-16/h3-8,11-12,15,22-23,25-40,42-48H,9-10,13-14H2,1-2H3/b8-5+/t15-,22-,23+,25-,26+,27+,28-,29+,30+,31+,32+,33+,34+,35+,36-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Cistanoside A possesses protective activities on CCl4 induced hepatotoxicity in mice, which is involved with increasing free radicals clearing activities, alleviating lipid-overoxidation damage, and improving respiratory chain function in mitochondria. |
| Targets | SOD |

Cistanoside A Dilution Calculator

Cistanoside A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.2488 mL | 6.2441 mL | 12.4883 mL | 24.9766 mL | 31.2207 mL |
| 5 mM | 0.2498 mL | 1.2488 mL | 2.4977 mL | 4.9953 mL | 6.2441 mL |
| 10 mM | 0.1249 mL | 0.6244 mL | 1.2488 mL | 2.4977 mL | 3.1221 mL |
| 50 mM | 0.025 mL | 0.1249 mL | 0.2498 mL | 0.4995 mL | 0.6244 mL |
| 100 mM | 0.0125 mL | 0.0624 mL | 0.1249 mL | 0.2498 mL | 0.3122 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 5-Aminouracil
Catalog No.:BCC8737
CAS No.:932-52-5
- 2-Hydroxybenzylamine
Catalog No.:BCN1803
CAS No.:932-30-9
- PPQ-102
Catalog No.:BCC5248
CAS No.:931706-15-9
- IOX2(Glycine)
Catalog No.:BCC2229
CAS No.:931398-72-0
- Tacalcitol monohydrate
Catalog No.:BCC1976
CAS No.:93129-94-3
- (R,E)-Deca-2-ene-4,6-diyne-1,8-diol
Catalog No.:BCN4476
CAS No.:931116-24-4
- (S,E)-Deca-2,9-diene-4,6-diyne-1,8-diol
Catalog No.:BCN1305
CAS No.:931114-98-6
- Ciprofloxacin hydrochloride
Catalog No.:BCC8915
CAS No.:93107-08-5
- Enrofloxacin
Catalog No.:BCC4657
CAS No.:93106-60-6
- Kaempferol 3-sophoroside-7-rhamnoside
Catalog No.:BCN1306
CAS No.:93098-79-4
- 8-O-Demethyl-7-O-methyl-3,9-dihydropunctatin
Catalog No.:BCN1307
CAS No.:93078-83-2
- R 59-022
Catalog No.:BCC7279
CAS No.:93076-89-2
- Boc-β-iodo-Ala-OMe
Catalog No.:BCC3052
CAS No.:93267-04-0
- CDDO-EA
Catalog No.:BCC5282
CAS No.:932730-51-3
- (±)-PPCC oxalate
Catalog No.:BCC7796
CAS No.:932736-91-9
- Iodoacetyl-LC-Biotin
Catalog No.:BCC3584
CAS No.:93285-75-7
- Monomethyl lithospermate
Catalog No.:BCN8124
CAS No.:933054-33-2
- 20-Hydroxy-3-oxo-28-lupanoic acid
Catalog No.:BCN4478
CAS No.:93372-87-3
- (+)-Taddol
Catalog No.:BCC8378
CAS No.:93379-49-8
- (S)-(-)-Atenolol
Catalog No.:BCC6633
CAS No.:93379-54-5
- 8-Epicrepiside E
Catalog No.:BCN7098
CAS No.:93395-30-3
- 8beta-(2-Hydroxy-2-methyl-3-oxobutyryloxy)glucozaluzanin C
Catalog No.:BCN6682
CAS No.:93395-31-4
- 2-Aminobenzimidazole
Catalog No.:BCC8547
CAS No.:934-32-7
- 2-Benzothiazolol
Catalog No.:BCC8557
CAS No.:934-34-9
[Transformation pathways in methanol of echinacoside, a principal effective ingredient of Cistanches Herba].[Pubmed:29945385]
Zhongguo Zhong Yao Za Zhi. 2018 Jun;43(11):2321-2325.
Echinacoside (ECH) is one of the active ingredients in Cistanche Herba and the principal effective component of Memoregain(c) as well. Moreover, a new agent namely Naoqing Zhiming tablet, derived from ECH has been licensed for clinical trials. However, the knowledge regarding the stability of is limited, till now, initiating a significant barrier for its further development along with the clinical trials. Herein, we aim to in depth characterize the transformation pattern of ECH in methanol. When ECH was stored in methanol, two primary products (P1 and P2) could be observed in HPLC chromatogram. A home-made automated fraction collector was configured via employing two 2-phase/6-port electronic valves to prepare P1 and P2. Following (1)H-NMR and LC-MS/MS assays, P1 and P2 were unambiguously identified as acteoside and Cistanoside A, respectively. Moreover, the existences of cis-ECH, cis-acteoside, and cis-Cistanoside A were claimed after careful analysis of the (1)H-NMR spectra of ECH, P1 and P2. Above all, the primary transformation pathways of ECH in methanol included methylation as well as hydrolysis, and mild transformation could also be initiated by cis/trans- configuration transferring for the caffeoyl group. The findings obtained in current study are envisioned to provide useful insight for the further development of ECH and the impurity detection of Naoqing Zhiming tablet. Moreover, the automated fraction collector configured in current study is able to serve as a versatile tool for the collection of signals-of-interest within phytochemical evaluations and impurity isolation.
[Study on quality evaluation of Dihuang (Rehmannia glutinosa) by two-dimension HPLC fingerprints and chemometrics methods].[Pubmed:29751715]
Zhongguo Zhong Yao Za Zhi. 2018 Apr;43(8):1667-1674.
The study is to establish the two-dimension HPLC fingerprints of Dihuang (Rehmannia glutinosa), by HPLC-PDA and HPLC-ELSD methods. The separations were performed on Waters Atlantis(R)T34.6 mmx 250 mm5 mumand Welch Ultimate(R)Hilic-NH(2)4.6 mmx 250 mm5 mumcolumns with the gradient elution of acetonitrile-0.01% phosphoric acid and acetonitrile-water, respectively. The chromatographic display wavelength for PDA detector was set at 203 nm. For HPLC-ELSD, the nebulizer was set as cooling mode, the drift tube temperature was set at 60 degrees C and the gas pressure was 35.0 psi. Based on similarity evaluation system for chromatographic fingerprint of traditional Chinese medicine, 26 and 10 chromatographic peaks were determined as common components for HPLC-PDA and HPLC-ELSD fingerprints, respectively. Chemometrics analyses, such as similarity analysis; cluster analysis and principal component analysis, were performed on the common peak areas in two-dimension fingerprints for 41 batches of Dihuang from multiple sources. The results showed that the HPLC-PDA fingerprint could distinguish dried rehmannia root between different sources, and HPLC-ELSD fingerprint could differentiate dried rehmannia root from prepared rehmannia root. The two-dimension fingerprints were established with advantages of a good degree of separation, abundant chemical information and multi-components identified including two nucleosides (adenosine and uridine)four iridoid glycosides (catalpa alcoholrehmaionoside Drehmaionoside A and leonuride)two phenylethanoid glycosides (acteoside and Cistanoside A) and nine sugars. The method is simple and practical, which could be used for the identification and quality assessment for Dihuang.
Comparison of the Chemical Profiles and Antioxidant Activities of Different Parts of Cultivated Cistanche deserticola Using Ultra Performance Liquid Chromatography-Quadrupole Time-of-Flight Mass Spectrometry and a 1,1-Diphenyl-2-picrylhydrazyl-Based Assay.[Pubmed:29156652]
Molecules. 2017 Nov 20;22(11). pii: molecules22112011.
In this study, a sensitive ultra-performance liquid chromatography-photodiode array coupled to quadruple time-of-flight mass (UPLC-PDA-Q/TOF-MS) method and a 1,1-diphenyl-2- picrylhydrazyl (DPPH)-based assay were used to determine the chemical constituents and screen the antioxidant activity profiles of the methanol extracts of different parts of cultivated Cistanche deserticola (C. deserticola). First, qualitative and quantitative chemical composition analyses of the different parts of cultivated C. deserticola were conducted. Obvious differences were observed between the chemical profiles and content distribution of phenylethanoid glycosides (PhGs) from the different cultivated C. deserticola parts. The average contents of the six PhGs parts varied from 4.91 to 72.56 mg/g DW (milligrams of extract per gram of plant dry weight) in the six different parts of Cistanche deserticola, displaying a significant decreasing trend from the bottom to the top of cultivated C. deserticola and the highest content in the stems. From the bottom to the top of the plant, the echinacoside and Cistanoside A content decreased and the 2 ' -acetylacteoside content increased. Second, an offline DPPH assay revealed that the total scavenging activities of all parts within the range of 20-500 mu g/mL increased in a concentration-dependent manner and that good antioxidant activities were found in all plant parts, particularly in the stems, which could be related to their higher PhG content. Additionally, a DPPH-UPLC-PDA method was successfully applied to rapidly screen the antioxidant profiles and antioxidant components of the different cultivated C. deserticola parts. According to the antioxidant profiles before and after the DPPH reaction, there were wide variations in the antioxidant activities of different cultivated C. deserticola parts. Moreover, the antioxidant profiles revealed the presence of major free radical scavengers identified as PhGs using UPLC-Q/TOF-MS. Finally, the established DPPH-UPLC-PDA method was reagent saving, rapid and feasible for correlating the chemical profile of traditional chinese medicines (TCMs) with their bioactivities without isolation and purification and may be used for multicomponent analysis of active substances in other foods and herbs. Therefore, to better harness C. deserticola resources, using this method to evaluate cultivated C. deserticola, a promising herb material with obvious antioxidant activity, is crucial.
Therapeutic Effect of Cistanoside A on Bone Metabolism of Ovariectomized Mice.[Pubmed:28125037]
Molecules. 2017 Jan 24;22(2). pii: molecules22020197.
Cistanoside A (Cis A), an active phenylethanoid glycoside isolated from Cistanche deserticola Y. C. Ma, has received our attention because of its possible role in the treatment of osteoporosis. In the present study, we evaluated the effects of Cis A on an ovariectomized (OVX) mice model and investigated its underlying molecular mechanisms of action. After 12 weeks of orally-administrated intervention, Cis A (20, 40 and 80 mg/kg body weight/day) exhibited significant antiosteoporotic effects on OVX mice, evidenced by enhanced bone strength, bone mineral density and improved trabecular bone microarchitecture. Meanwhile, the activities of bone resorption markers, including tartrate-resistant acid phosphatase (TRAP), deoxypyridinoline (DPD) and cathepsin K, were decreased, and the bioactivity of bone formation marker alkaline phosphatase (ALP) was increased. Mechanistically, Cis A inhibited the expression of TNF-receptor associated factor 6 (TRAF6), an upstream molecule that is shared by both nuclear factor kappa-light chain enhancer of activated B cells (NF-kappaB) and phosphatidylinositol 3-kinase (PI3K)/Akt pathways and subsequently suppressed the levels of receptor activators of nuclear factor kappaB ligand (RANKL), downregulated the expression of NF-kappaB and upregulated osteoprotegerin (OPG), PI3K and Akt, which means Cis A possessed antiosteoporotic activity in ovariectomized mice via TRAF6-mediated NF-kappaB inactivation and PI3K/Akt activation. Put together, we present novel findings that Cis A, by downregulating TRAF6, coordinates the inhibition of NF-kappaB and stimulation of PI3K/Akt pathways to promote bone formation and prevent bone resorption. These data demonstrated the potential of Cis A as a promising agent for the treatment of osteoporosis disease.
Protective effect of Cistanchis A on ethanol-induced damage in primary cultured mouse hepatocytes.[Pubmed:27544551]
Biomed Pharmacother. 2016 Oct;83:1071-1079.
Cistanoside A (C. A) was one of phenylethanol glycosides isolated from Cistanche deserticola, a tonic in traditional Chinese medicine. In our previous research, we demonstrated that Cistanoside A (C. A) possess the protective activities on CCl4 induced hepatotoxicity in mice, such as increasing free radicals clearing activities, alleviating lipid-overoxidation damage, and improving respiratory chain function in mitochondria. Meanwhile, our previous research also demonstrated C.A possess protective activities on alcohol induced hepatotoxicity in mice, shown in ameliorate the hepatic function indices, lightening steatosis and inflammatory infiltration, increasing free radicals clearing activities, alleviating lipid-overoxidation damage, and alleviating energy metabolism in mitochondria. The aim of this research was to evaluate the effects of Cistanoside A (C. A) on ethanol-induced damage in primary cultured mouse hepatocytes, and probe into the mechanism related. Using fluorescent staining, flow cytometer, immunohistochemistry analysis, and Western blotting, we demonstrated that C.A could enhance the survival rate of the primary cultured hepatocytes, alleviate apoptosis and necrosis, the mechanism was involved with enhance the expression of apoptosis inhibition factor bcl-2, and inhibition the expression of immediate early genes c-fos.
[Identification of Chinese Traditional Medicine Cistanches Herba from Different Places by HPLC-ESI-MS and FTIR Methods].[Pubmed:26197602]
Guang Pu Xue Yu Guang Pu Fen Xi. 2015 Apr;35(4):1056-61.
Five samples of Cistanches Herba from different places were analyzed by HPLC-ESI-MS and FTIR methods. The effective compositions in Cistanches Herba including Cistanoside A, echinacoside, acteoside , isoacteoside, 2'-actylacteoside, cistanoside C and tubluoside B were determined by HPLC-MS. The common peak ratio and variant peak ratio were calculated by FTIR spectroscopy of the five samples and the dual index sequence of common peak ratio and variant peak ratio were established. The results showed that the evaluation results of the samples by the two methods were the same. The general fake plant Cynomorii Herba could be identified by FTIR. HPLC-ESI-MS, which has high sensitivity and rapid determination procedure, can be used to evaluate quality of Cistanches Herba by quantitative analysis of the primary compositions. FTIR is a non-destructive analysis method. without complicated extraction and separation procedures to the samples. The absorption strength and the absorption shape were the synergistic effect of the functional groups and the nestification of the components in Cistanches Herba. The provided method has some advantages such as rapid analysis process, good reproducibility, non-destructive, small quantity of sample, simple treatment, good specificity, low-cost and environment-friendly. The method meets the trend of complex analysis and whole analysis for the Chinese medicines. Combination of FTIR and HPLC-ESI-MS was a good method for identification and evaluation of quality of Chinese medicines.
Chemical and genetic discrimination of Cistanches Herba based on UPLC-QTOF/MS and DNA barcoding.[Pubmed:24854031]
PLoS One. 2014 May 22;9(5):e98061.
Cistanches Herba (Rou Cong Rong), known as "Ginseng of the desert", has a striking curative effect on strength and nourishment, especially in kidney reinforcement to strengthen yang. However, the two plant origins of Cistanches Herba, Cistanche deserticola and Cistanche tubulosa, vary in terms of pharmacological action and chemical components. To discriminate the plant origin of Cistanches Herba, a combined method system of chemical and genetic--UPLC-QTOF/MS technology and DNA barcoding--were firstly employed in this study. The results indicated that three potential marker compounds (isomer of campneoside II, cistanoside C, and Cistanoside A) were obtained to discriminate the two origins by PCA and OPLS-DA analyses. DNA barcoding enabled to differentiate two origins accurately. NJ tree showed that two origins clustered into two clades. Our findings demonstrate that the two origins of Cistanches Herba possess different chemical compositions and genetic variation. This is the first reported evaluation of two origins of Cistanches Herba, and the finding will facilitate quality control and its clinical application.
Effects of 2-aminoindan-2-phosphonic acid treatment on the accumulation of salidroside and four phenylethanoid glycosides in suspension cell culture of Cistanche deserticola.[Pubmed:21243361]
Plant Cell Rep. 2011 Apr;30(4):665-74.
2-Aminoindan-2-phosphonic acid (AIP), a specific competitive phenylalanine ammonia lyase (PAL) inhibitor was applied to a suspension cell culture of Cistanche deserticola. The effects of AIP treatment on cell growth, PAL activity, contents and yields of total phenolic compound, salidroside and four phenylethanoid glycosides (PheGs) are investigated. The results demonstrated that, 0.5 and 2.0 muM AIP treatments had similar effects on the measurements investigated in this study. AIP treatment resulted in significant decreases in PAL activity, total phenolic compounds content, and PheGs content. Linear regression analysis showed that PAL activity had a high correlation coefficient with the total phenolic compound content and the four PheGs contents. Total PAL activity-time area under curve (AUC) had a high correlation coefficient with the total phenolic compound yield and the yields of five tested compounds in untreated cell samples. In AIP-treated cells, total PAL activity-time AUC retained a high correlation with the total phenolic compound yield and the yields of three tested compounds, echinacoside, acteoside, and tubuloside A, but not salidroside and Cistanoside A. The difference could be caused by the different biosynthetic origins of each of the tested compounds. These results demonstrate the important role of PAL in the biosynthesis of PheGs in the suspension cell culture of C. deserticola.
[Structure-activity relationships of phenylethanoid glycosides in plants of Cistanche salsa on antioxidative activity].[Pubmed:19873735]
Zhong Yao Cai. 2009 Jul;32(7):1067-9.
OBJECTIVE: To study the structure-activity relationships of phenylethanoid glycosides in plants of Cistanche salsa on antioxidative activity. METHODS: By the assay systems of DPPH*, the antioxidant activity of six phenylethanoid glycosides from plants of Cistanche salsa was determined to investigate the relationship between the antioxidant activities and phenylethanoid glycosides's structural characteristics. RESULTS: The antioxidative activity of phenylethanoid glycosides was variant with dose-dependent effect. The sequence of the strength of the antioxidative activity of the six components was shown to be 2'-Acetylacteoside > Acteoside > or = Tubuloside B > or = Isoacteoside > Echinacoside > Cistanoside A. CONCLUSION: The antioxidative activity of phenylethanoid glycosides is related to the number of phenolic hydroxyl, steric hindrance, 2-acetyl on the middle glucopyranose, and the location of phenolic hydroxyl. Additionally, it may be related to the alpha, beta-unsaturated ketone of phenl-2-propenoyl.
Isolation and purification of phenylethanoid glycosides from Cistanche deserticola by high-speed counter-current chromatography.[Pubmed:26059151]
Food Chem. 2008 May 15;108(2):702-10.
Five phenylethanoid glycosides (PhGs), echinacoside, Cistanoside A, acteoside, isoacteoside and 2'-acetylacteoside, were isolated and purified from Cistanche deserticola for the first time by high-speed counter-current chromatography (HSCCC) using two biphasic systems, one consisting of ethyl acetate-ethanol-water (5:0.5:4.5, v/v/v) and another of ethyl acetate-n-butanol-ethanol-water (0.5:0.5:0.1:1, v/v/v/v). A total of 28.5mg of echinacoside, 18.4mg of Cistanoside A, 14.6mg of acteoside, 30.1mg of isoacteoside and 25.2mg of 2'-acetylacteoside were purified from 1412mg of the n-butanol extract of C. deserticola, each at over 92.5% purity as determined by HPLC. The structures were identified by their retention time, UV, LC-ESI-MS in the negative ion mode, and confirmed by NMR experiments. The characteristic LC-ESI-MS(n) fragmentation pattern of the five compounds is discussed, and found to be a very specific and useful tool for the structural identification of PhGs from this important medicinal plant.
[Determination of phenylethanoid glycosides from Cistanche deserticola in spring and autumn with LC-MS].[Pubmed:15272779]
Zhong Yao Cai. 2004 Mar;27(3):175-7.
OBJECTIVE: To have a contrast study on phenylethanoid glycosides from Cistanche deserticola Y. C. Ma collected in different seasons. METHODS: LC/MS method has been applied for the analysis of four kinds of phenylethanoid glycosides compunds (echinacoside, acteoside, Cistanoside A and 2'-acetylacteoside) from Cistanche deserticola Y. C. Ma in spring and autumn. RESULTS: According to the special MS spectra and HPLC chromatogram, this four kinds of phenylethanoid glycosides compounds were detected in each Cistanche deserticola Y. C. Ma, but the content is considerable different except the acteoside. CONCLUSION: The content of phenylethanoid glycosides from Cistanche deserticola Y. C. Ma in different seasons has a difference from each other, the quality of Cistanche deserticola Y. C. Ma is also different.
[A study on quality standard for herba cistanches].[Pubmed:12512425]
Zhongguo Zhong Yao Za Zhi. 2000 Jun;25(6):359-61.
OBJECTIVE: To establish the quality standard for Herba Cistanches. METHOD: A qualitative identification method by TLC was established for five kinds of active components(acteoside, echinacoside, Cistanoside A, betaine and mannitol) and RP-HPLC was used to quantify the acteoside contents. RESULTS: Qualitative and quantitative analyses were carried out for fourteen kinds of Herba Cistanches and eleven kinds of Yinpian. This method is accurate, reliable and of good separability and reproducibility. CONCLUSION: This method can be applied as standard for the quality control of cistanche deserticola.
[Analysis of phenylethanoid glycosides in the extract of herba Cistanchis by LC/ESI-MS/MS].[Pubmed:11218862]
Yao Xue Xue Bao. 2000 Nov;35(11):839-42.
AIM: To analyze the phenylethanoid glycosides in Cistanche deserticola Y. D. Ma and its alternatives. METHODS: An HPLC/MS/MS method has been developed for the analysis of seven kinds of phenylethanoid glycosides in Cistanche deserticola Y. D. Ma, C. salsa (C. A. Mey) G. Beck and C. tubulosa (Schenk) R. Wight. The [M - H]- ions were observed for five standards and Cistanche extracts. The glycosidic linkages, the core, and the attached sugar (s) of the phenylethanoid glycosides can be determined from the collision-induced dissociation spectra of the molecular. RESULTS: Seven kinds of phenylethanoid glycosides (echinacoside, acteoside, cisacteoside, isoacteoside, 2'-acetylacteoside, Cistanoside A, osmanthuside B) in Cistanche deserticola Y. D. Ma, six kinds (echinacoside, acteoside, cisacteoside, isoacteoside, 2'-acetylacteoside and Cistanoside A) in C. salsa (C. A. Mey) G. Beck and five kinds (echinacoside, acteoside, cisacteoside, isoacteoside and 2'-acetylacteoside) in C. tubulosa (Schenk) R. Wight were detected. CONCLUSION: The difference of the relative distribution of these phenylethanoid glycosides in each extract was found out. Phenylethanoid glycosides are the specific constituents in Cistanchis, which can be used to distinguish different species in Genus Cistanchis.
Inhibition of nitric oxide by phenylethanoids in activated macrophages.[Pubmed:10913595]
Eur J Pharmacol. 2000 Jul 14;400(1):137-44.
Nitric oxide (NO) is one of the pro-inflammatory molecules. Some phenylethanoids have been previously shown to possess anti-inflammatory effects. Seven phenylethanoids from the stems of Cistanche deserticola, viz. isoacteoside, tubuloside B, acteoside, 2'-O-acetylacteoside, echinacoside, Cistanoside A and tubuloside A, were tested for their effect on NO radical generation by activated murine macrophages. At the concentration of 100-200 microM, all the phenylethanoids reduced (6.3-62.3%) nitrite accumulation in lipopolysaccharide (0.1 microgram/ml)-stimulated J774.1 cells. At 200 microM, they inhibited by 32.2-72.4% nitrite accumulation induced by lipopolysaccharide (0.1 microgram/ml)/interferon-gamma (100 U/ml) in mouse peritoneal exudate macrophages. However, these compounds did not affect the expression of inducible nitric oxide (iNOS) mRNA, the iNOS protein level, or the iNOS activity in lipopolysaccharide-stimulated J774.1 cells. Instead, they showed a clear scavenging effect (6.9-43.9%) at the low concentrations of 2-10 microM of about 12 microM nitrite generated from an NO donor, 1-propanamine-3-hydroxy-2-nitroso-1-propylhydrazino (PAPA NONOate). These results indicate that the phenylethanoids have NO radical-scavenging activity, which possibly contributes to their anti-inflammatory effects.


