PACAP 1-38Potent stimulator of adenylyl cyclase CAS# 137061-48-4 |
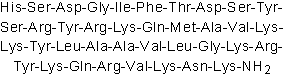
- Q-VD-OPh hydrate
Catalog No.:BCC1125
CAS No.:1135695-98-5
- Z-VAD-FMK
Catalog No.:BCC1126
CAS No.:187389-52-2
- Boc-D-FMK
Catalog No.:BCC1128
CAS No.:187389-53-3,634911-80-1
- Z-DEVD-FMK
Catalog No.:BCC1137
CAS No.:210344-95-9
- Z-VDVAD-FMK
Catalog No.:BCC1138
CAS No.:N/A
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 137061-48-4 | SDF | Download SDF |
| PubChem ID | 131954544 | Appearance | Powder |
| Formula | C203H331N63O53S | M.Wt | 4534.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Pituitary Adenylate Cyclase-Activating Polypeptide 1-38 | ||
| Solubility | H2O Peptide Solubility and Storage Guidelines: 1. Calculate the length of the peptide. 2. Calculate the overall charge of the entire peptide according to the following table: 3. Recommended solution: | ||
| Sequence | HSDGIFTDSYSRYRKQMAVKKYLAAVLGKR (Modifications: Lys-38 = C-terminal amide) | ||
| Chemical Name | 4-[[2-[[1-[[1-[[1-[[1-[[1-[[1-[[1-[[1-[[1-[[1-[[6-amino-1-[[5-amino-1-[[1-[[1-[[1-[[6-amino-1-[[6-amino-1-[[1-[[1-[[1-[[1-[[1-[[1-[[2-[[6-amino-1-[[1-[[1-[[6-amino-1-[[5-amino-1-[[1-[[1-[[6-amino-1-[[4-amino-1-[(1,6-diamino-1-oxohexan-2-yl)amino]-1,4-dioxobutan-2-yl]amino]-1-oxohexan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-1,5-dioxopentan-2-yl]amino]-1-oxohexan-2-yl]amino]-3-(4-hydroxyphenyl)-1-oxopropan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-1-oxohexan-2-yl]amino]-2-oxoethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-1-oxopropan-2-yl]amino]-1-oxopropan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-(4-hydroxyphenyl)-1-oxopropan-2-yl]amino]-1-oxohexan-2-yl]amino]-1-oxohexan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-1-oxopropan-2-yl]amino]-4-methylsulfanyl-1-oxobutan-2-yl]amino]-1,5-dioxopentan-2-yl]amino]-1-oxohexan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-3-(4-hydroxyphenyl)-1-oxopropan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-(4-hydroxyphenyl)-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-carboxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxobutan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-3-methyl-1-oxopentan-2-yl]amino]-2-oxoethyl]amino]-3-[[2-[[2-amino-3-(1H-imidazol-4-yl)propanoyl]amino]-3-hydroxypropanoyl]amino]-4-oxobutanoic acid | ||
| SMILES | CCC(C)C(C(=O)NC(CC1=CC=CC=C1)C(=O)NC(C(C)O)C(=O)NC(CC(=O)O)C(=O)NC(CO)C(=O)NC(CC2=CC=C(C=C2)O)C(=O)NC(CO)C(=O)NC(CCCNC(=N)N)C(=O)NC(CC3=CC=C(C=C3)O)C(=O)NC(CCCNC(=N)N)C(=O)NC(CCCCN)C(=O)NC(CCC(=O)N)C(=O)NC(CCSC)C(=O)NC(C)C(=O)NC(C(C)C)C(=O)NC(CCCCN)C(=O)NC(CCCCN)C(=O)NC(CC4=CC=C(C=C4)O)C(=O)NC(CC(C)C)C(=O)NC(C)C(=O)NC(C)C(=O)NC(C(C)C)C(=O)NC(CC(C)C)C(=O)NCC(=O)NC(CCCCN)C(=O)NC(CCCNC(=N)N)C(=O)NC(CC5=CC=C(C=C5)O)C(=O)NC(CCCCN)C(=O)NC(CCC(=O)N)C(=O)NC(CCCNC(=N)N)C(=O)NC(C(C)C)C(=O)NC(CCCCN)C(=O)NC(CC(=O)N)C(=O)NC(CCCCN)C(=O)N)NC(=O)CNC(=O)C(CC(=O)O)NC(=O)C(CO)NC(=O)C(CC6=CNC=N6)N | ||
| Standard InChIKey | UFTCZKMBJOPXDM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C203H331N63O53S/c1-18-109(12)162(262-156(279)99-230-170(290)147(95-157(280)281)255-193(313)149(100-267)259-168(288)124(211)93-119-97-224-103-231-119)198(318)257-145(88-114-40-20-19-21-41-114)191(311)266-163(113(16)270)199(319)258-148(96-158(282)283)190(310)261-151(102-269)194(314)253-144(92-118-59-67-123(274)68-60-118)188(308)260-150(101-268)192(312)243-134(51-38-83-227-202(220)221)180(300)251-142(90-116-55-63-121(272)64-56-116)186(306)242-132(49-36-81-225-200(216)217)176(296)237-127(44-24-31-76-206)173(293)245-137(70-72-153(213)276)182(302)246-138(73-85-320-17)171(291)233-112(15)167(287)263-159(106(6)7)195(315)247-130(47-27-34-79-209)175(295)238-129(46-26-33-78-208)177(297)252-143(91-117-57-65-122(273)66-58-117)187(307)249-140(87-105(4)5)184(304)234-110(13)165(285)232-111(14)166(286)264-160(107(8)9)197(317)256-139(86-104(2)3)169(289)229-98-155(278)235-126(43-23-30-75-205)172(292)239-133(50-37-82-226-201(218)219)179(299)250-141(89-115-53-61-120(271)62-54-115)185(305)241-128(45-25-32-77-207)174(294)244-136(69-71-152(212)275)181(301)240-135(52-39-84-228-203(222)223)183(303)265-161(108(10)11)196(316)248-131(48-28-35-80-210)178(298)254-146(94-154(214)277)189(309)236-125(164(215)284)42-22-29-74-204/h19-21,40-41,53-68,97,103-113,124-151,159-163,267-274H,18,22-39,42-52,69-96,98-102,204-211H2,1-17H3,(H2,212,275)(H2,213,276)(H2,214,277)(H2,215,284)(H,224,231)(H,229,289)(H,230,290)(H,232,285)(H,233,291)(H,234,304)(H,235,278)(H,236,309)(H,237,296)(H,238,295)(H,239,292)(H,240,301)(H,241,305)(H,242,306)(H,243,312)(H,244,294)(H,245,293)(H,246,302)(H,247,315)(H,248,316)(H,249,307)(H,250,299)(H,251,300)(H,252,297)(H,253,314)(H,254,298)(H,255,313)(H,256,317)(H,257,318)(H,258,319)(H,259,288)(H,260,308)(H,261,310)(H,262,279)(H,263,287)(H,264,286)(H,265,303)(H,266,311)(H,280,281)(H,282,283)(H4,216,217,225)(H4,218,219,226)(H4,220,221,227)(H4,222,223,228) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Endogenous neuropeptide showing considerable homology with vasoactive intestinal peptide (VIP) but with a greater potency for stimulation of adenylyl cyclase. |

PACAP 1-38 Dilution Calculator

PACAP 1-38 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Walsuralactam A
Catalog No.:BCN6734
CAS No.:1370556-82-3
- Episyringaresinol 4'-O-β-D-glncopyranoside
Catalog No.:BCC8957
CAS No.:137038-13-2
- Pseudoginsenoside Rh2
Catalog No.:BCC8353
CAS No.:1370264-16-6
- PRT062607 Hydrochloride
Catalog No.:BCC1869
CAS No.:1370261-97-4
- 3',4'-Di-O-acetyl-2',6'-di-O-p-coumaroylastragalin
Catalog No.:BCN6610
CAS No.:137018-33-8
- Amprolium HCl
Catalog No.:BCC4626
CAS No.:137-88-2
- L-Ascorbyl 6-palmitate
Catalog No.:BCC4915
CAS No.:137-66-6
- Lidocaine
Catalog No.:BCC1084
CAS No.:137-58-6
- Calcium pantothenate
Catalog No.:BCN8503
CAS No.:137-08-6
- Sophoraflavanone I
Catalog No.:BCN6863
CAS No.:136997-69-8
- Sophoraflavanone H
Catalog No.:BCN6864
CAS No.:136997-68-7
- (-)-Syringaresnol-4-O-beta-D-apiofuranosyl-(1->2)-beta-D-glucopyranoside
Catalog No.:BCN1578
CAS No.:136997-64-3
- Alprenolol hydrochloride
Catalog No.:BCC7490
CAS No.:13707-88-5
- Pimecrolimus
Catalog No.:BCC4703
CAS No.:137071-32-0
- Tolfenamic Acid
Catalog No.:BCC4438
CAS No.:13710-19-5
- (±)-Marmesin
Catalog No.:BCN3618
CAS No.:13710-70-8
- BETP
Catalog No.:BCC6286
CAS No.:1371569-69-5
- Toddalosin
Catalog No.:BCN6194
CAS No.:137182-37-7
- Spinorphin
Catalog No.:BCC2349
CAS No.:137201-62-8
- Sodium Orthovanadate
Catalog No.:BCC3856
CAS No.:13721-39-6
- Voriconazole
Catalog No.:BCC2275
CAS No.:137234-62-9
- Dodoviscin A
Catalog No.:BCN3927
CAS No.:1372527-25-7
- Dodoviscin H
Catalog No.:BCN3918
CAS No.:1372527-39-3
- Dodoviscin I
Catalog No.:BCN3926
CAS No.:1372527-40-6
Effects of peptide molecular mass and PEG chain length on the vasoreactivity of VIP and PACAP(1-38) in pegylated phospholipid micelles.[Pubmed:15350692]
Peptides. 2004 Aug;25(8):1253-8.
Bioactive properties of certain amphipathic peptides are amplified when self-associated with sterically stabilized micelles (SSM) composed of polyethylene glycol (PEG)-conjugated phospholipids. The purpose of this study was to determine the effects of amphipathic peptide molecular mass and PEG chain length on vasoreactivity evoked by vasoactive intestinal peptide (VIP), a 28-amino acid neuropeptide, and pituitary adenylate cyclase-activating peptide(1-38) (PACAP(1-38)), a 38-amino acid neuropeptide, associated with PEGylated phospholipid micelles in vivo. Both peptides were incubated for 2 h with SSM composed of PEG with molecular mass of 2000 or 5000 grafted onto distearoyl-phosphatidylethanolamine (DSPE-PEG2000 or DSPE-PEG5000) before use. We found that regardless of peptide molecular mass, PEG chain length had no significant effects on peptide-SSM interactions. Using intravital microscopy, VIP associated with DSPE-PEG5000 SSM or DSPE-PEG2000 SSM incubated at 25 degrees C evoked similar vasodilation in the intact hamster cheek pouch microcirculation. Likewise, PACAP(1-38)-induced vasodilation was PEG chain length-independent. However, SSM-associated PACAP(1-38) evoked significantly smaller vasodilation than that evoked by SSM-associated VIP (P < 0.05) at 25 degrees C. When the incubation temperature was increased to 37 degrees C, SSM-associated PACAP(1-38)-induced vasodilation was now similar to that of SSM-associated VIP. This response was associated with a corresponding increase in alpha-helix content of both peptides in the presence of phospholipids. Collectively, these data indicate that for a larger amphipathic peptide, such as PACAP(1-38), greater kinetic energy or longer incubation period is required to optimize peptide-SSM interactions and amplify peptide bioactivity in vivo.
Interactions of VIP, secretin and PACAP(1-38) with phospholipids: a biological paradox revisited.[Pubmed:12678867]
Curr Pharm Des. 2003;9(12):1005-12.
Vasoactive intestinal peptide (VIP), secretin and pituitary adenylate cyclase-activating peptide(1-38)(PACAP(1-38)) are widely distributed amphipathic mammalian neuropeptides that exert diverse biological effects in target tissues located distant from their site of release. However, the half-life of exogenously-administered VIP, secretin and PACAP(1-38) in the bloodstream is relatively short (minutes) due to rapid degradation and inactivation. This seemingly paradoxical behavior suggests the presence of an innate system(s) that protects the peptides from degradation in vivo. To this end, VIP, secretin and PACAP(1-38) express distinct biophysical properties that once released may protect them from degradation in biological fluids. They self-aggregate at low (nanomolar) concentrations and interact avidly with biomimetic phospholipid monolayers and bilayers at physiological concentrations. The latter evokes conformational transition of the VIP, secretin and PACAP(1-38) molecules from predominantly random coil in aqueous solution to alpha-helix, the preferred peptide conformation for receptor interaction, in phospholipids. These features increase peptide stability and amplify bioactivity in vivo. Collectively, these data suggest the presence of an endogenous targeted delivery platform for VIP, secretin and PACAP(1-38). This innate system may constitute a novel molecular recognition paradigm that could also apply to other amphipathic neuropeptides. Importantly, the distinct behavior of VIP, secretin and PACAP(1-38) in the presence of phospholipids could be exploited to develop novel, long-acting therapeutic formulations of these peptides.
Pituitary adenylate cyclase-activating polypeptide (PACAP(1-38)) enhances N-methyl-D-aspartate receptor function and brain-derived neurotrophic factor expression via RACK1.[Pubmed:12524444]
J Biol Chem. 2003 Mar 14;278(11):9630-8.
We recently identified a novel mechanism for modulation of the phosphorylation state and function of the N-methyl-d-aspartate (NMDA) receptor via the scaffolding protein RACK1. We found that RACK1 binds both the NR2B subunit of the NMDA receptor and the nonreceptor protein-tyrosine kinase, Fyn. RACK1 inhibits Fyn phosphorylation of NR2B and decreases NMDA receptor-mediated currents in CA1 hippocampal slices (Yaka, R., Thornton, C., Vagts, A. J., Phamluong, K., Bonci, A., and Ron, D. (2002) Proc. Natl. Acad. Sci. U. S. A. 99, 5710-5715). Here, we identified the signaling cascade by which RACK1 is released from the NMDA receptor complex and identified the consequences of the dissociation. We found that activation of the cAMP/protein kinase A pathway in hippocampal slices induced the release of RACK1 from NR2B and Fyn. This resulted in the induction of NR2B phosphorylation and the enhancement of NMDA receptor-mediated activity via Fyn. We identified the neuropeptide, pituitary adenylate cyclase activating polypeptide (PACAP(1-38)), as a ligand that induced phosphorylation of NR2B and enhanced NMDA receptor potentials. Finally, we found that activation of the cAMP/protein kinase A pathway induced the movement of RACK1 to the nuclear compartment in dissociated hippocampal neurons. Nuclear RACK1 in turn was found to regulate the expression of brain-derived neurotrophic factor induced by PACAP(1-38). Taken together our results suggest that activation of adenylate cyclase by PACAP(1-38) results in the release of RACK1 from the NMDA receptor and Fyn. This in turn leads to NMDA receptor phosphorylation, enhanced activity mediated by Fyn, and to the induction of brain-derived neurotrophic factor expression by RACK1.
Phospholipids modulate the biophysical properties and vasoactivity of PACAP-(1--38).[Pubmed:12235038]
J Appl Physiol (1985). 2002 Oct;93(4):1377-83.
The purpose of this study was to elucidate the interactions between pituitary adenylate cyclase-activating peptide (PACAP)-(1--38) and phospholipids in vitro and to determine whether these phenomena modulate, in part, the vasorelaxant effects of the peptide in the intact peripheral microcirculation. We found that the critical micellar concentration of PACAP-(1--38) was 0.4-0.9 microM. PACAP-(1--38) significantly increased the surface tension of a dipalmitoylphosphatidylcholine monolayer and underwent conformational transition from predominantly random coil in saline to alpha-helix in the presence of distearoyl-phosphatidylethanolamine-polyethylene glycol (molecular mass of 2,000 Da) sterically stabilized phospholipid micelles (SSM) (P < 0.05). Using intravital microscopy, we found that aqueous PACAP-(1--38) evoked significant concentration-dependent vasodilation in the intact hamster cheek pouch that was significantly potentiated when PACAP-(1--38) was associated with SSM (P < 0.05). The vasorelaxant effects of aqueous PACAP-(1--38) were mediated predominantly by PACAP type 1 (PAC(1)) receptors, whereas those of PACAP-(1--38) in SSM predominantly by PACAP/vasoactive intestinal peptide type 1 and 2 (VPAC(1)/VPAC(2)) receptors. Collectively, these data indicate that PACAP-(1--38) self-associates and interacts avidly with phospholipids in vitro and that these phenomena amplify peptide vasoactivity in the intact peripheral microcirculation.
The 38-amino-acid form of pituitary adenylate cyclase-activating polypeptide induces neurite outgrowth in PC12 cells that is dependent on protein kinase C and extracellular signal-regulated kinase but not on protein kinase A, nerve growth factor receptor tyrosine kinase, p21(ras) G protein, and pp60(c-src) cytoplasmic tyrosine kinase.[Pubmed:9730914]
Mol Pharmacol. 1998 Sep;54(3):547-58.
The 38-amino-acid isoform of pituitary adenylate cyclase-activating polypeptide (PACAP38) elicits a robust outgrowth of neurites in cultured PC12 cells. Initiation of neurite outgrowth occurs within 4-8 hr after the addition of PACAP38. Treatment with PACAP38 does not elicit collateral activation of p140(trk) nerve growth factor receptor tyrosine kinase activity, nor is it associated with tyrosine phosphorylation of suc1-associated neurotrophic factor target, a selective target of neurotrophin tyrosine kinase receptors. Coadministration of epidermal growth factor with PACAP38 elicits an enhanced response. Induction of neurites is also observed on the addition of PACAP38 to dominant negative Src and Ras PC12 cell variants. PACAP38 stimulates extracellular signal-regulated kinase (Erk) activity >10-fold within 5 min, and the effect is augmented by cotreatment with epidermal growth factor. Pretreatment with the cAMP-dependent protein kinase-selective inhibitor, H-89, is ineffective as an antagonist of PACAP38-induced neurite outgrowth, whereas down-regulation of protein kinase C (PKC) by phorbol ester or incubation with PKC-selective inhibitors GF109203X and calphostin C effectively blocks PACAP38-stimulated neurite formation. Stimulation of Erk activity is inhibited by incubation with PD90859, a pharmacological antagonist of the threonine/tyrosine dual-specificity Erk. Inhibition of ligand-stimulated Erk activation prevents PACAP38-induced neurite outgrowth. Collectively, these findings indicate that PACAP38-stimulated neuritogenesis requires PKC and Erk activation but is independent of cAMP-dependent protein kinase, nerve growth factor receptor tyrosine kinase, p21(ras) G protein, and pp60(c-src) cytoplasmic tyrosine kinase.
Pituitary adenylate cyclase-activating polypeptide increases [Ca2]i in rat gonadotrophs through an inositol trisphosphate-dependent mechanism.[Pubmed:7907085]
J Biol Chem. 1994 Feb 25;269(8):5680-6.
Pituitary adenylate cyclase-activating polypeptide (PACAP) increases cAMP production and stimulates hormone release from a variety of anterior pituitary cells. However, in anterior pituitary gonadotrophs PACAP stimulates oscillations in cytosolic free Ca2+ concentration ([Ca2+]i) that appear to be independent of cAMP. To study the mechanisms involved in this response, we used the patch-clamp technique to microperfuse various agents into single rat gonadotrophs while monitoring [Ca2+]i with microfluorometry. Extracellular application of PACAP to single gonadotrophs stimulated high amplitude (> 1 microM) oscillations in [Ca2+]i, which were blocked by intracellular application of GDP beta S (guanosine 5'-O-2-thiodiphosphate), indicating the involvement of a G-protein. To identify the intracellular messenger(s) involved, we microperfused gonadotrophs with cAMP, inositol 1,4,5-trisphosphate (Ins(1,4,5)P3), or heparin, an antagonist of the Ins(1,4,5)P3 receptor. A high concentration of cAMP (100 microM) had no significant effect on basal [Ca2+]i and did not alter the PACAP-stimulated Ca2+ response. Heparin, but not its inactive isoform, completely blocked the PACAP-stimulated increase in [Ca2+]i, while Ins(1,4,5)P3 stimulated oscillations in [Ca2+]i very similar to those observed in response to PACAP. These results strongly suggest that PACAP mobilizes Ca2+ through an Ins(1,4,5)P3-dependent mechanism. The fact that PACAP stimulates two signaling pathways in pituitary cells could substantially enhance the signaling potential of this hypothalamic peptide.


