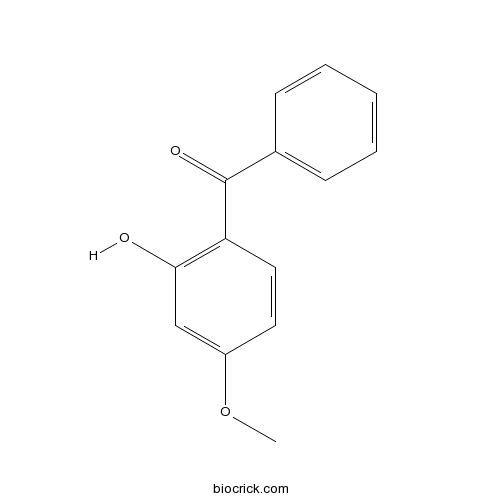
OxybenzoneCAS# 131-57-7 |
- Daptomycin
Catalog No.:BCC1057
CAS No.:103060-53-3
- Nelarabine
Catalog No.:BCC1072
CAS No.:121032-29-9
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Clofarabine
Catalog No.:BCC1078
CAS No.:123318-82-1
- Ifosfamide
Catalog No.:BCC1164
CAS No.:3778-73-2
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 131-57-7 | SDF | Download SDF |
| PubChem ID | 4632 | Appearance | Powder |
| Formula | C14H12O3 | M.Wt | 228.24 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Benzophenone 3 | ||
| Solubility | DMSO : ≥ 100 mg/mL (438.14 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (2-hydroxy-4-methoxyphenyl)-phenylmethanone | ||
| SMILES | COC1=CC(=C(C=C1)C(=O)C2=CC=CC=C2)O | ||
| Standard InChIKey | DXGLGDHPHMLXJC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H12O3/c1-17-11-7-8-12(13(15)9-11)14(16)10-5-3-2-4-6-10/h2-9,15H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Oxybenzone Dilution Calculator

Oxybenzone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.3814 mL | 21.9068 mL | 43.8135 mL | 87.6271 mL | 109.5338 mL |
| 5 mM | 0.8763 mL | 4.3814 mL | 8.7627 mL | 17.5254 mL | 21.9068 mL |
| 10 mM | 0.4381 mL | 2.1907 mL | 4.3814 mL | 8.7627 mL | 10.9534 mL |
| 50 mM | 0.0876 mL | 0.4381 mL | 0.8763 mL | 1.7525 mL | 2.1907 mL |
| 100 mM | 0.0438 mL | 0.2191 mL | 0.4381 mL | 0.8763 mL | 1.0953 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Oxybenzone(Eusolex 4360; Escalol 567) is an organic compound used in sunscreens; provides broad-spectrum ultraviolet coverage, including UVB and short-wave UVA rays.
References:
[1]. Oxybenzone, From Wikipedia
- N-Acetylneuraminic acid
Catalog No.:BCN2204
CAS No.:131-48-6
- Pimpinellin
Catalog No.:BCN6168
CAS No.:131-12-4
- Dimethyl phthalate
Catalog No.:BCN6167
CAS No.:131-11-3
- alpha-Yohimbine
Catalog No.:BCN6166
CAS No.:131-03-3
- VU 0360172 hydrochloride
Catalog No.:BCC6141
CAS No.:1309976-62-2
- 15-Methoxymkapwanin
Catalog No.:BCN6498
CAS No.:1309920-99-7
- MRS 4062 triethylammonium salt
Catalog No.:BCC6134
CAS No.:1309871-50-8
- Sephin1
Catalog No.:BCC3980
CAS No.:13098-73-2
- (±)-CPSI 1306
Catalog No.:BCC6161
CAS No.:1309793-47-2
- H 89 2HCl
Catalog No.:BCC4997
CAS No.:130964-39-5
- Marumoside A
Catalog No.:BCN7702
CAS No.:1309604-34-9
- Wittifuran X
Catalog No.:BCN4794
CAS No.:1309478-07-6
- Meptyldinocap
Catalog No.:BCC5468
CAS No.:131-72-6
- 8-Epiloganic acid
Catalog No.:BCC8956
CAS No.:82509-41-9
- Boldenone undecylenate
Catalog No.:BCC8896
CAS No.:13103-34-9
- GNF179 Metabolite
Catalog No.:BCC5176
CAS No.:1310455-86-7
- NB-598
Catalog No.:BCC1786
CAS No.:131060-14-5
- Tubastatin A HCl
Catalog No.:BCC3877
CAS No.:1310693-92-5
- NVP-BGJ398 phosphate
Catalog No.:BCC1814
CAS No.:1310746-10-1
- Miltipolone
Catalog No.:BCN3222
CAS No.:131086-61-8
- 5,7-Dichlorokynurenic acid
Catalog No.:BCC6592
CAS No.:131123-76-7
- BIX 02565
Catalog No.:BCC4303
CAS No.:1311367-27-7
- mGlu2 agonist
Catalog No.:BCC1745
CAS No.:1311385-32-6
- Sodium Demethylcantharidate
Catalog No.:BCN8394
CAS No.:13114-29-9
Metabolism of oxybenzone in a hairy root culture: Perspectives for phytoremediation of a widely used sunscreen agent.[Pubmed:26736174]
J Hazard Mater. 2016 Apr 5;306:230-236.
Oxybenzone (OBZ), known as Benzophenone-3, is a commonly used UV filter in sun tans and skin protectants, entering aquatic systems either directly during recreational activities or indirectly through wastewater treatment plants discharge. To study the potential degradation capacity of plants for OBZ in phytotreatment, a well-established hairy root culture (Armoracia rusticana) was treated with OBZ. More than 20% of spiked OBZ (100muM) was eliminated from the medium by hairy roots after 3h of exposure. Two metabolites were identified as Oxybenzone-glucoside (OBZ-Glu) and Oxybenzone-(6-O-malonyl)-glucoside (OBZ-Mal-Glu) by LC-MS/MS and TOF-MS. Formation of these metabolites was confirmed by enzymatic synthesis, as well as enzymatic and alkaline hydrolysis. Incubation with O-glucosyltransferase (O-GT) extracted from roots formed OBZ-Glu; whereas beta-d-Glucosidase hydrolyzed OBZ-Glu. However, alkaline hydrolysis led to cleavage of OBZ-Mal-Glu and yielded OBZ-Glu. In the hairy root culture, an excretion of OBZ-Glu into the growth medium was observed while the corresponding OBZ-Mal-Glu remained stored in root cells over the incubation time. We propose that metabolism of Oxybenzone in plants involves initial conjugation with glucose to form OBZ-Glu followed by malonylation to yield OBZ-Mal-Glu. To our best knowledge this first finding presenting the potential of plants to degrade benzophenone type UV filters by phytoremediation.
Simultaneous Determination of Octinoxate, Oxybenzone, and Octocrylene in a Sunscreen Formulation Using Validated Spectrophotometric and Chemometric Methods.[Pubmed:26525239]
J AOAC Int. 2015 Sep-Oct;98(5):1215-25.
Accurate, reliable, and sensitive spectrophotometric and chemometric methods were developed for simultaneous determination of octinoxate (OMC), Oxybenzone (OXY), and octocrylene (OCR) in a sunscreen formulation without prior separation steps, including derivative ratio spectra zero crossing (DRSZ), double divisor ratio spectra derivative (DDRD), mean centering ratio spectra (MCR), and partial least squares (PLS-2). With the DRSZ technique, the UV filters could be determined in the ranges of 0.5-13.0, 0.3-9.0, and 0.5-9.0 mug/mL at 265.2, 246.6, and 261.8 nm, respectively. By utilizing the DDRD technique, UV filters could be determined in the above ranges at 237.8, 241.0, and 254.2 nm, respectively. With the MCR technique, the UV filters could be determined in the above ranges at 381.7, 383.2, and 355.6 nm, respectively. The PLS-2 technique successfully quantified the examined UV filters in the ranges of 0.5-9.3, 0.3-7.1, and 0.5-6.9 mug/mL, respectively. All the methods were validated according to the International Conference on Harmonization guidelines and successfully applied to determine the UV filters in pure form, laboratory-prepared mixtures, and a sunscreen formulation. The obtained results were statistically compared with reference and reported methods of analysis for OXY, OMC, and OCR, and there were no significant differences with respect to accuracy and precision of the adopted techniques.
Photodynamics of oxybenzone sunscreen: Nonadiabatic dynamics simulations.[Pubmed:27544106]
J Chem Phys. 2016 Aug 21;145(7):074308.
Herein we have used combined static electronic structure calculations and "on-the-fly" global-switching trajectory surface-hopping dynamics simulations to explore the photochemical mechanism of Oxybenzone sunscreen. We have first employed the multi-configurational CASSCF method to optimize minima, conical intersections, and minimum-energy reaction paths related to excited-state intramolecular proton transfer (ESIPT) and excited-state decays in the (1)pipi( *), (1)npi( *), and S0 states (energies are refined at the higher MS-CASPT2 level). According to the mapped potential energy profiles, we have identified two ultrafast excited-state deactivation pathways for the initially populated (1)pipi( *) system. The first is the diabatic ESIPT process along the (1)pipi( *) potential energy profile. The generated (1)pipi( *) keto species then decays to the S0 state via the keto (1)pipi( *)/gs conical intersection. The second is internal conversion to the dark (1)npi( *) state near the (1)pipi( *) /(1)npi( *) crossing point in the course of the diabatic (1)pipi( *) ESIPT process. Our following dynamics simulations have shown that the ESIPT and (1)pipi( *) --> S0 internal conversion times are 104 and 286 fs, respectively. Finally, our present work demonstrates that in addition to the ESIPT process and the (1)pipi( *) --> S0 internal conversion in the keto region, the (1)pipi( *) --> (1)npi( *) internal conversion in the enol region plays as well an important role for the excited-state relaxation dynamics of Oxybenzone.
Chlorination of oxybenzone: Kinetics, transformation, disinfection byproducts formation, and genotoxicity changes.[Pubmed:27085067]
Chemosphere. 2016 Jul;154:521-527.
UV filters are a kind of emerging contaminant, and their transformation behavior in water treatment processes has aroused great concern. In particular, toxic products might be produced during reaction with disinfectants during the disinfection process. As one of the most widely used UV filters, Oxybenzone has received significant attention, because its transformation and toxicity changes during chlorine oxidation are a concern. In our study, the reaction between Oxybenzone and chlorine followed pseudo-first-order and second-order kinetics. Three transformation products were detected by LC-MS/MS, and the stability of products followed the order of tri-chloro-methoxyphenoyl > di-chlorinated Oxybenzone > mono-chlorinated Oxybenzone. Disinfection byproducts (DBPs) including chloroform, trichloroacetic acid, dichloroacetic acid and chloral hydrate were quickly formed, and increased at a slower rate until their concentrations remained constant. The maximum DBP/Oxybenzone molar yields for the four compounds were 12.02%, 6.28%, 0.90% and 0.23%, respectively. SOS/umu genotoxicity test indicated that genotoxicity was highly elevated after chlorination, and genotoxicity showed a significantly positive correlation with the response of tri-chloro-methoxyphenoyl. Our results indicated that more genotoxic transformation products were produced in spite of the elimination of Oxybenzone, posing potential threats to drinking water safety. This study shed light on the formation of DBPs and toxicity changes during the chlorination process of Oxybenzone.


