OrlistatLipase inhibitor for obesity treatment CAS# 96829-58-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 96829-58-2 | SDF | Download SDF |
| PubChem ID | 3034010 | Appearance | Powder |
| Formula | C29H53NO5 | M.Wt | 495.73 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Tetrahydrolipstatin; Ro-18-0647 | ||
| Solubility | DMSO : ≥ 100 mg/mL (201.72 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
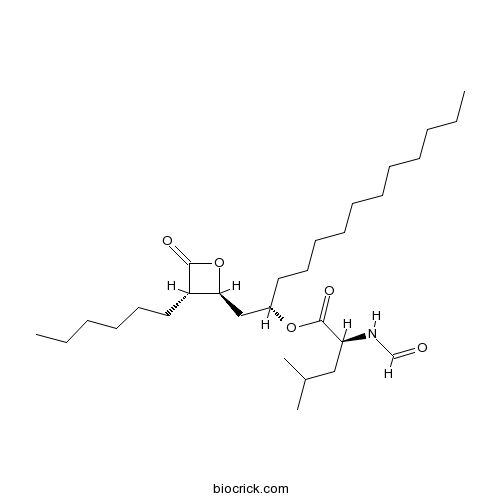
| Chemical Name | [(2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-2-yl] (2S)-2-formamido-4-methylpentanoate | ||
| SMILES | CCCCCCCCCCCC(CC1C(C(=O)O1)CCCCCC)OC(=O)C(CC(C)C)NC=O | ||
| Standard InChIKey | AHLBNYSZXLDEJQ-FWEHEUNISA-N | ||
| Standard InChI | InChI=1S/C29H53NO5/c1-5-7-9-11-12-13-14-15-16-18-24(34-29(33)26(30-22-31)20-23(3)4)21-27-25(28(32)35-27)19-17-10-8-6-2/h22-27H,5-21H2,1-4H3,(H,30,31)/t24-,25-,26-,27-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Hypolipemic pancreatic, gastric and carboxylester lipase inhibitor. Exhibits no activity at phospholipase A2, liver esterase, trypsin and chymotrypsin. Inhibits the thioesterase domain of fatty acid synthase, leading to cell cycle arrest at the G1/S boundary in vitro. Prevents the absorption of approximately one third of fat from food and exhibits progastrokinetic, antiobesity and antihypercholesterolemic activity in vivo. |

Orlistat Dilution Calculator

Orlistat Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0172 mL | 10.0861 mL | 20.1723 mL | 40.3445 mL | 50.4307 mL |
| 5 mM | 0.4034 mL | 2.0172 mL | 4.0345 mL | 8.0689 mL | 10.0861 mL |
| 10 mM | 0.2017 mL | 1.0086 mL | 2.0172 mL | 4.0345 mL | 5.0431 mL |
| 50 mM | 0.0403 mL | 0.2017 mL | 0.4034 mL | 0.8069 mL | 1.0086 mL |
| 100 mM | 0.0202 mL | 0.1009 mL | 0.2017 mL | 0.4034 mL | 0.5043 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Orlistat (also known as tetrahydrolipstatin) is a drug designed to treat obesity.Orlistat is the saturated derivative of lipstatin, a potent natural inhibitor of pancreatic lipases isolated from the bacterium Streptomyces toxytricini.
- Chlorovaltrate K
Catalog No.:BCN7126
CAS No.:96801-92-2
- (±)-McN 5652
Catalog No.:BCC7267
CAS No.:96795-89-0
- Chlorisondamine diiodide
Catalog No.:BCC6885
CAS No.:96750-66-2
- [D-Arg1,D-Phe5,D-Trp7,9,Leu11]-Substance P
Catalog No.:BCC7211
CAS No.:96736-12-8
- Rabdoserrin A
Catalog No.:BCN8041
CAS No.:96685-01-7
- Viscidulin III tetraacetate
Catalog No.:BCN4515
CAS No.:96684-81-0
- Decuroside I
Catalog No.:BCN3909
CAS No.:96638-79-8
- Ocinaplon
Catalog No.:BCC6167
CAS No.:96604-21-6
- 3beta-Hydroxylanosta-8,24-diene-21-al
Catalog No.:BCN3329
CAS No.:96574-03-7
- Salvianolic acid A
Catalog No.:BCN5951
CAS No.:96574-01-5
- Szechenyine
Catalog No.:BCN2601
CAS No.:96562-88-8
- 3,3'-[Iminobis(methylene)]bis-2(3H)furanone
Catalog No.:BCN1295
CAS No.:96562-86-6
- Przewaquinone C
Catalog No.:BCN3003
CAS No.:96839-29-1
- Indatraline hydrochloride
Catalog No.:BCC7123
CAS No.:96850-13-4
- 8beta-Tigloyloxycostunolide
Catalog No.:BCN7115
CAS No.:96850-21-4
- Maoecrystal B
Catalog No.:BCN4516
CAS No.:96850-29-2
- Maoecrystal A
Catalog No.:BCN5407
CAS No.:96850-30-5
- XCC
Catalog No.:BCC7890
CAS No.:96865-83-7
- XAC
Catalog No.:BCC7600
CAS No.:96865-92-8
- VIP (guinea pig)
Catalog No.:BCC5725
CAS No.:96886-24-7
- Nyasol
Catalog No.:BCN7579
CAS No.:96895-25-9
- 1,2-Bis(4'-methyl-2,2'-bipyridin-4-yl)ethane
Catalog No.:BCC8414
CAS No.:96897-04-0
- Cyproheptadine hydrochloride
Catalog No.:BCC5161
CAS No.:969-33-5
- Artanin
Catalog No.:BCN4517
CAS No.:96917-26-9
Gelidium elegans Regulates the AMPK-PRDM16-UCP-1 Pathway and Has a Synergistic Effect with Orlistat on Obesity-Associated Features in Mice Fed a High-Fat Diet.[Pubmed:28358328]
Nutrients. 2017 Mar 30;9(4). pii: nu9040342.
The incidence of obesity is rising at an alarming rate throughout the world and is becoming a major public health concern with incalculable social and economic costs. Gelidium elegans (GENS), also previously known as Gelidium amansii, has been shown to exhibit anti-obesity effects. Nevertheless, the mechanism by which GENS is able to do this remains unclear. In the present study, our results showed that GENS prevents high-fat diet (HFD)-induced weight gain through modulation of the adenosine monophosphate-activated protein kinase (AMPK)-PR domain-containing16 (PRDM16)-uncoupling protein-1 (UCP-1) pathway in a mice model. We also found that GENS decreased hyperglycemia in mice that had been fed a HFD compared to corresponding controls. We also assessed the beneficial effect of the combined treatment with GENS and Orlistat (a Food and Drug Administration-approved obesity drug) on obesity characteristics in HFD-fed mice. We found that in HFD-fed mice, the combination of GENS and Orlistat is associated with more significant weight loss than Orlistat treatment alone. Moreover, our results demonstrated a positive synergistic effect of GENS and Orlistat on hyperglycemia and plasma triglyceride level in these animals. Thus, we suggest that a combination therapy of GENS and Orlistat may positively influence obesity-related health outcomes in a diet-induced obese population.
A strategy for dual inhibition of the proteasome and fatty acid synthase with belactosin C-orlistat hybrids.[Pubmed:28236510]
Bioorg Med Chem. 2017 Jun 1;25(11):2901-2916.
The proteasome, a validated cellular target for cancer, is central for maintaining cellular homeostasis, while fatty acid synthase (FAS), a novel target for numerous cancers, is responsible for palmitic acid biosynthesis. Perturbation of either enzymatic machine results in decreased proliferation and ultimately cellular apoptosis. Based on structural similarities, we hypothesized that hybrid molecules of belactosin C, a known proteasome inhibitor, and Orlistat, a known inhibitor of the thioesterase domain of FAS, could inhibit both enzymes. Herein, we describe proof-of-principle studies leading to the design, synthesis and enzymatic activity of several novel, beta-lactone-based, dual inhibitors of these two enzymes. Validation of dual enzyme targeting through activity-based proteome profiling with an alkyne probe modeled after the most potent inhibitor, and preliminary serum stability studies of selected derivatives are also described. These results provide proof of concept for dual targeting of the proteasome and fatty acid synthase-thioesterase (FAS-TE) enabling a new approach for the development of drug-candidates with potential to overcome resistance.
Effect of orlistat on periostin, adiponectin, inflammatory markers and ultrasound grades of fatty liver in obese NAFLD patients.[Pubmed:28260907]
Ther Clin Risk Manag. 2017 Feb 20;13:139-149.
Orlistat is recommended in the treatment of obesity, which is an independent risk factor for nonalcoholic fatty liver disease (NAFLD). The reported findings of Orlistat in NAFLD are divisive. Recently, periostin is identified as an important regulatory molecule in the pathogenesis of obesity-induced fatty liver. Therefore, this study aimed to evaluate the potential effects of Orlistat in the treatment of NAFLD. A 16-week prospective observational study was conducted, with obese NAFLD patient (n=77) receiving Orlistat (120 mg capsules, three times a day) with hypocaloric diet or hypocaloric diet only. Grades of fatty liver were determined using ultrasound (US) echogenicity of liver; serum levels of periostin, adiponectin, tumor necrosis factor (TNF)-alpha and interleukin-6 were determined using ELISA kits at 0 and 16 weeks. Correlations of US grades of fatty liver with these biomarkers were also determined. Orlistat significantly reversed the US grades of fatty liver (P=0.016), decreased serum levels of periostin (P=0.030) and TNF-alpha (P=0.040), and increased serum adiponectin levels (P<0.001) when compared with hypocaloric diet only. Serum interleukin-6 levels were not found to be significantly different in both groups after the treatment. In the Orlistat group, the degree of reduction in grades of fatty liver was found to be positively correlated with the changes in serum levels of periostin (rs=0.306, P=0.041) and adiponectin (rs=0.314, P=0.036), whereas the associations were insignificant with the change in serum levels of TNF-alpha (rs=0.053, P=0.729). Mild gastrointestinal side effects (20%) were reported in the Orlistat group. In conclusion, Orlistat is effective in the treatment of NAFLD patients without fibrosis. This study demonstrated a positive association between the reduction of fatty infiltration in the liver and the changes in serum levels of periostin and adiponectin in obese NAFLD patients.
Orlistat accelerates gastric emptying and attenuates GIP release in healthy subjects.[Pubmed:19109408]
Am J Physiol Gastrointest Liver Physiol. 2009 Mar;296(3):G482-9.
Orlistat, an inhibitor of digestive lipases, is widely used for the treatment of obesity. Previous reports on the effect of orally ingested Orlistat together with a meal on gastric emptying and secretion of gut peptides that modulate postprandial responses are controversial. We investigated the effect of ingested Orlistat on gastric emptying and plasma responses of gut peptides in response to a solid mixed meal with a moderate energy load. In healthy subjects, gastric emptying was determined using scintigraphy and studies were performed without and with 120 mg of Orlistat in pellet form in random order. Orlistat shortened t lag and t half and decreased the area under the gastric emptying curve. Orlistat significantly attenuated the secretion of glucose-dependent insulinotropic polypeptide (GIP) but did not alter the plasma responses of cholecystokinin (CCK), glucagon-like peptide-1 (GLP-1), pancreatic polypeptide (PP), and insulin. There was no peptide YY (PYY) response. Area under the curve of gastric emptying was positively correlated with integrated secretion of GIP (r=0.786) in Orlistat and was negatively correlated with integrated plasma response of GLP-1 (r=-0.75) in control experiments, implying that inhibition of fat absorption modifies determinants of gastric emptying of a meal. Orlistat administered similar to its use in obesity treatment accelerates gastric emptying of a solid mixed meal with a moderate energy load and profoundly attenuates release of GIP without appreciably altering plasma responses of CCK, GLP-1, and PP. Since GIP is being implemented in the development of obesity, its role in weight control attained by Orlistat awaits further investigation.
Orlistat is a novel inhibitor of fatty acid synthase with antitumor activity.[Pubmed:15026345]
Cancer Res. 2004 Mar 15;64(6):2070-5.
One of the fundamental principles of pharmacology is that most drugs have side effects. Although considerable attention is paid to detrimental side effects, drugs can also have beneficial side effects. Given the time and expense of drug development, it would be particularly exciting if a systematic method could be applied to reveal all of the activities, including the unappreciated actions, of a potential drug. The present study takes the first step along this path. An activity-based proteomics strategy was used to simultaneously identify targets and screen for their inhibitors in prostate cancer. Orlistat, a Food and Drug Administration-approved drug used for treating obesity, was included in this screen. Surprisingly, we find a new molecular target and a potential new application for Orlistat. Orlistat is a novel inhibitor of the thioesterase domain of fatty acid synthase, an enzyme strongly linked to tumor progression. By virtue of its ability to inhibit fatty acid synthase, Orlistat halts tumor cell proliferation, induces tumor cell apoptosis, and inhibits the growth of PC-3 tumors in nude mice.
The lipase inhibitor tetrahydrolipstatin binds covalently to the putative active site serine of pancreatic lipase.[Pubmed:1899234]
J Biol Chem. 1991 Feb 5;266(4):2021-7.
Tetrahydrolipstatin (THL) is a selective inhibitor of fat absorption. In animal models, it has anti-obesity and anti-hypercholesterolemic activity and is presently in clinical trials for these indications. THL binds covalently to pancreatic lipase. Complete inhibition of lipolytic activity is obtained concomitant with the incorporation of 1 mol of THL/mol of enzyme. Pancreatic lipase is the best studied lipase, but published results concerning its catalytic mechanism are still controversial. In order to learn more about the inhibitory mechanism of THL, a selective lipase inhibitor interacting at or near the catalytic site, and therefore, to obtain more information on the catalytic mechanism of lipase, we have determined the amino acid residue to which THL is bound. After proteolytic degradation of porcine pancreatic lipase inhibited with radioactively labeled THL, the labeled peptides were isolated and analyzed by quantitative amino acid analysis, N-terminal sequencing, and by mass spectrometry with fast atom bombardment ionization. The data clearly show that THL is bound as an ester to the serine 152 of the lipase.


