OBAAPhospholipase A2 inhibitor CAS# 221632-26-4 |
- Cefditoren Pivoxil
Catalog No.:BCC4898
CAS No.:117467-28-4
- Cefoselis
Catalog No.:BCC4092
CAS No.:122841-10-5
- Balofloxacin
Catalog No.:BCC4892
CAS No.:127294-70-6
- Pefloxacin Mesylate Dihydrate
Catalog No.:BCC5089
CAS No.:149676-40-4
- Tinidazole
Catalog No.:BCC4866
CAS No.:19387-91-8
- Toltrazuril
Catalog No.:BCC4870
CAS No.:69004-03-1
Quality Control & MSDS
Number of papers citing our products

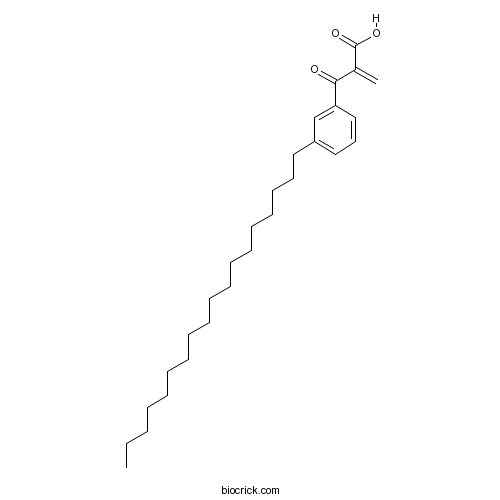
Chemical structure

3D structure
| Cas No. | 221632-26-4 | SDF | Download SDF |
| PubChem ID | 20981629 | Appearance | Powder |
| Formula | C28H44O3 | M.Wt | 428.65 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in DMSO with gentle warming | ||
| Chemical Name | 2-(3-octadecylbenzoyl)prop-2-enoic acid | ||
| SMILES | CCCCCCCCCCCCCCCCCCC1=CC=CC(=C1)C(=O)C(=C)C(=O)O | ||
| Standard InChIKey | OZIGHNVFXHALAU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C28H44O3/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-20-25-21-19-22-26(23-25)27(29)24(2)28(30)31/h19,21-23H,2-18,20H2,1H3,(H,30,31) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A potent inhibitor of phospholipase A2 (IC50 = 70 nM). Reduces bronchospasm in guinea pigs in vivo. |

OBAA Dilution Calculator

OBAA Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3329 mL | 11.6645 mL | 23.3291 mL | 46.6581 mL | 58.3226 mL |
| 5 mM | 0.4666 mL | 2.3329 mL | 4.6658 mL | 9.3316 mL | 11.6645 mL |
| 10 mM | 0.2333 mL | 1.1665 mL | 2.3329 mL | 4.6658 mL | 5.8323 mL |
| 50 mM | 0.0467 mL | 0.2333 mL | 0.4666 mL | 0.9332 mL | 1.1665 mL |
| 100 mM | 0.0233 mL | 0.1166 mL | 0.2333 mL | 0.4666 mL | 0.5832 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- L-Menthol
Catalog No.:BCN4971
CAS No.:2216-51-5
- Boron tributoxide
Catalog No.:BCN8281
CAS No.:688-74-4
- 2,6-Dimethoxy-1-acetonylquinol
Catalog No.:BCN5052
CAS No.:2215-96-5
- 1-Benzyl-1H-indazol-3-ol
Catalog No.:BCC8458
CAS No.:2215-63-6
- Stigmastane-3,6-dione
Catalog No.:BCN5051
CAS No.:22149-69-5
- Hopane-3beta,22-diol
Catalog No.:BCN4852
CAS No.:22149-65-1
- Kaempferol 3-gentiobioside
Catalog No.:BCN3345
CAS No.:22149-35-5
- 11-Hydroxytabersonine
Catalog No.:BCN5050
CAS No.:22149-28-6
- 2 beta-Hydroxykolavelool
Catalog No.:BCN4671
CAS No.:221466-42-8
- 2-Oxokolavelool
Catalog No.:BCN4672
CAS No.:221466-41-7
- Fischeria A
Catalog No.:BCN3779
CAS No.:221456-63-9
- Cytochalasin D
Catalog No.:BCN5049
CAS No.:22144-77-0
- Conocarpan
Catalog No.:BCN5053
CAS No.:221666-27-9
- Methylswertianin
Catalog No.:BCN8505
CAS No.:22172-17-4
- Zotarolimus(ABT-578)
Catalog No.:BCC5481
CAS No.:221877-54-9
- Macrocarpal N
Catalog No.:BCN5811
CAS No.:221899-21-4
- L-Canavanine sulfate
Catalog No.:BCC6746
CAS No.:2219-31-0
- Lyn peptide inhibitor
Catalog No.:BCC5895
CAS No.:222018-18-0
- Pyrantel Pamoate
Catalog No.:BCC4958
CAS No.:22204-24-6
- Naproxen
Catalog No.:BCC9091
CAS No.:22204-53-1
- Dihydroseselin
Catalog No.:BCN8258
CAS No.:2221-66-1
- Dehydroglaucine
Catalog No.:BCN2548
CAS No.:22212-26-6
- Cucurbitacin I
Catalog No.:BCC2439
CAS No.:2222-07-3
- Thalrugosaminine
Catalog No.:BCN7745
CAS No.:22226-73-9
The role of arachidonic acid/cyclooxygenase cascade, phosphodiesterase IV and Rho-kinase in H2S-induced relaxation in the mouse corpus cavernosum.[Pubmed:28501682]
Pharmacol Rep. 2017 Aug;69(4):610-615.
BACKGROUND: Penile corpus cavernosum is an extremely vascularized tissue and cavernosal smooth muscle tone is regulated by the balance between contractile and relaxant factor. We investigated the possible role of arachidonic acid/cyclooxygenase cascade, phosphodiesterase IV (PDEIV) and Rho-kinase in exogenous hydrogen sulfide (H2S)-induced relaxation in mouse corpus cavernosum. METHODS: The relaxant response to H2S (NaHS as exogenous H2S; 1-1000muM) were obtained in isolated mouse corpus cavernosum tissues which pre-contracted by phenylephrine (5muM). The effects of 4-(4-octadecylphenyl)-4-oxobutenoic acid (OBAA; 10muM), a selective phospholipase A2 (PLA2) inhibitor, indomethacin (1muM), a non-selective cyclooxygenase (COX) inhibitor, baicalein (10muM), a lipoxygenase (LOX) inhibitor, and proadifen (10muM), cytochrome P450 inhibitor, on the relaxant responses to H2S were investigated. Furthermore, the effects of theophylline (500muM) and rolipram (1muM), a non-selective and selective PDEIV inhibitor, and fasudil (3muM), a specific Rho-kinase inhibitor, were studied on H2S-induced relaxation. RESULTS: H2S-induced relaxations were significantly reduced by OBAA, indomethacin and proadifen but not baicalein. Furthermore, theophylline, rolipram and fasudil reduced H2S-induced relaxations. CONCLUSION: These results suggest that PLA2, COX, cytochrome P450, PDEIV and Rho-kinase pathway may involve in H2S-induced relaxation in mouse corpus cavernosum tissues.
Ciproxifan, a histamine H3 receptor antagonist and inverse agonist, presynaptically inhibits glutamate release in rat hippocampus.[Pubmed:28132918]
Toxicol Appl Pharmacol. 2017 Mar 15;319:12-21.
Ciproxifan is an H3 receptor antagonist and inverse agonist with antipsychotic effects in several preclinical models; its effect on glutamate release has been investigated in the rat hippocampus. In a synaptosomal preparation, ciproxifan reduced 4-aminopyridine (4-AP)-evoked Ca(2+)-dependent glutamate release and cytosolic Ca(2+) concentration elevation but did not affect the membrane potential. The inhibitory effect of ciproxifan on 4-AP-evoked glutamate release was prevented by the Gi/Go-protein inhibitor pertussis toxin and Cav2.2 (N-type) and Cav2.1 (P/Q-type) channel blocker omega-conotoxin MVIIC, but was not affected by the intracellular Ca(2+)-release inhibitors dantrolene and CGP37157. Furthermore, the phospholipase A2 (PLA2) inhibitor OBAA, prostaglandin E2 (PGE2), PGE2 subtype 2 (EP2) receptor antagonist PF04418948, and extracellular signal-regulated kinase (ERK) inhibitor FR180204 eliminated the inhibitory effect of ciproxifan on glutamate release. Ciproxifan reduced the 4-AP-evoked phosphorylation of ERK and synapsin I, a presynaptic target of ERK. The ciproxifan-mediated inhibition of glutamate release was prevented in synaptosomes from synapsin I-deficient mice. Moreover, ciproxifan reduced the frequency of miniature excitatory postsynaptic currents without affecting their amplitude in hippocampal slices. Our data suggest that ciproxifan, acting through the blockade of Gi/Go protein-coupled H3 receptors present on hippocampal nerve terminals, reduces voltage-dependent Ca(2+) entry by diminishing PLA2/PGE2/EP2 receptor pathway, which subsequently suppresses the ERK/synapsin I cascade to decrease the evoked glutamate release.
Quantum dot-NBD-liposome luminescent probes for monitoring phospholipase A2 activity.[Pubmed:24173659]
Anal Bioanal Chem. 2013 Dec;405(30):9729-37.
In this paper we describe the fabrication and characterization of new liposome encapsulated quantum dot-fluorescence resonance energy transfer (FRET)-based probes for monitoring the enzymatic activity of phospholipase A2. To fabricate the probes, luminescent CdSe/ZnS quantum dots capped with trioctylphosphine oxide (TOPO) ligands were incorporated into the lipid bilayer of unilamellar liposomes with an average diameter of approximately 100 nm. Incorporating TOPO capped quantum dots in liposomes enabled their use in aqueous solution while maintaining their hydrophobicity and excellent photophysical properties. The phospholipid bilayer was labeled with the fluorophore NBD C6-HPC (2-(6-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)hexanoyl-1-hexa decanoyl-sn-glycero-3-phosphocholine). The luminescent quantum dots acted as FRET donors and the NBD dye molecules acted as FRET acceptors. The probe response was based on FRET interactions between the quantum dots and the NBD dye molecules. The NBD dye molecules were cleaved and released to the solution in the presence of the enzyme phospholipase A2. This led to an increase of the luminescence of the quantum dots and to a corresponding decrease in the fluorescence of the NBD molecules, because of a decrease in FRET efficiency between the quantum dots and the NBD dye molecules. Because the quantum dots were not attached covalently to the phospholipids, they did not hinder the enzyme activity as a result of steric effects. The probes were able to detect amounts of phospholipase A2 as low as 0.0075 U mL(-1) and to monitor enzyme activity in real time. The probes were also used to screen phospholipase A2 inhibitors. For example, we found that the inhibition efficiency of MJ33 (1-hexadecyl-3-(trifluoroethyl)-sn-glycero-2-phosphomethanol) was higher than that of OBAA (3-(4-octadecyl)benzoylacrylic acid).
Distinct receptors and different transduction mechanisms for ATP and adenosine at the frog motor nerve endings.[Pubmed:12956724]
Eur J Neurosci. 2003 Sep;18(5):1254-64.
Corelease of ATP with ACh from motor endings suggests a physiological role for ATP in synaptic transmission. We previously showed that, on skeletal muscle, ATP directly inhibited ACh release via presynaptic P2 receptors. The receptor identification (P2X or P2Y) and its transduction mechanism remained, however, unknown. In the present study using the voltage-clamp technique we analyzed the properties of presynaptic ATP receptors and subsequent effector mechanisms. ATP or adenosine presynaptically depressed multiquantal end-plate currents, with longer latency for ATP action. ATPgammaS, agonist at P2X receptors, or Bz-ATP, agonist at P2X7 receptors, were ineffective. The action of ATP was prevented by suramin and unchanged by PPADS or TNP-ATP, antagonists of P2X receptors, or RB-2, a blocker of certain P2Y receptors. The depressant action of ATP was reproduced by UTP, metabotropic P2Y receptor agonist. Pertussis toxin (PTX), antagonist of Gi/o-proteins, and inhibitors of phosphatidylcholine specific PLC (D609) and PKC (staurosporine or chelerythrine) prevented the effect of ATP while blockers of PLA2 (OBAA) and COX (aspirin or indomethacin) attenuated it. Inhibitors of phosphatidylinositide-specific PLC (U73122), guanylylcyclase (ODQ), PKA (Rp-cAMPS) or PLD (1-butanol) did not affect the action of ATP. No inhibitor of second messengers (except PTX) changed the action of adenosine. Our data indicate, for motor nerve endings, the existence of inhibitory P2Y receptors coupled to multiple intracellular cascades including phosphatidylinositide-specific PLC/PKC/PLA2/COX. This divergent presynaptic P2 signalling (unlike the single effector mechanism for P1 receptors) could provide feedback inhibition of transmitter release and perhaps be involved in presynaptic plasticity.
Regulation of pharyngeal motility by FMRFamide and related peptides in the medicinal leech, Hirudo medicinalis.[Pubmed:12491073]
Invert Neurosci. 1999-2000;4(1):41-53.
The medicinal leech possesses FMRFamide-like immunoreactivity in neural processes and somata associated with the pharynx and pharyngeal ganglia. The pharynx possessed about 25 immunoreactive somata; about half of the approximately 20 neurons of the pharyngeal ganglia were immunoreactive. We provide brief descriptions of several neurons located in the first neuromere of the subesophageal ganglion involved in controlling pharyngeal motility. Double-labeling experiments indicate that one of these cells, named Swallow neuron 1 (SW1), contains a FMRFamide-like peptide. Stimulation of SW1 caused the mouth to open and the pharynx to dilate. Upon termination of SW1 stimulation, the mouth closed, and a peristaltic wave progressed from the mouth down the length of the pharynx. Stimulation of SW1 did not produce 1:1 postsynaptic potentials in pharyngeal muscle cells. Thus, SW1 is apparently not a motor neuron. The pharynx responded to application of FMRFamide and related peptides by producing a series of 20- to 35-s phasic contractions superimposed upon an increase in basal tonus. The peptide-induced response was quantified by measuring increases in basal tonus, peak tension, and integrated area. Although there were some differences in the order of potency depending upon which parameter was considered, the approximate order of potency of RFamide peptides tested was: pQDPFLRFamide > or = FMRFamide approximately YGGFMRFamide > or = YMRFamide approximately FLRFamide approximately GGKYMRFamide approximately YLRFamide > leucomyosuppressin approximately perisulfakinin. Except for differences in potency, each of the RFamide peptides produced similar contractile waveforms. FMRFamide-induced responses were reduced by the protein kinase C inhibitor bisindolylmaleimide I (10 microM), the nonspecific protein kinase inhibitor H-7 (50 microM), and were increased by the protein phosphatase inhibitor okadaic acid (1 microM). However, the FMRFamide-induced response was unaffected by the protein kinase A inhibitor H-89 (1 microM), the phosphodiesterase inhibitor theophylline (1 mM), the phospholipase A(2) inhibitor OBAA (0.1 microM) or the cation channel blocker amiloride (100 microM).
Roles for nitric oxide and arachidonic acid in the induction of heterosynaptic cerebellar LTD.[Pubmed:11201073]
Neuroreport. 2001 Jan 22;12(1):133-6.
In cerebellar slices conjunctive pairing of parallel fibre (PF) stimulation with depolarization of Purkinje cells (PCs) induces a long-term depression (LTD) of PF synaptic transmission that spreads to unpaired PF inputs to the same cell. Inhibitors of NO synthase (7-nitro-indazole), soluble guanylate cyclase (ODQ) and PKG (KT5823) all prevented depression at each of two independent PF pathways to a single PC. Inhibition of NOS also unmasked a platelet activating factor (PAF)-mediated synaptic potentiation of possible presynaptic origin. LTD was also prevented by the phospholipase A2 inhibitor OBAA but was rescued by co-perfusion with arachidonic acid. We conclude that NO and diffusible products of phospholipase A2 metabolism are potential mediators of the spread of cerebellar plasticity at the single cell level.
The Ginkgo biloba extract (EGb 761) protects and rescues hippocampal cells against nitric oxide-induced toxicity: involvement of its flavonoid constituents and protein kinase C.[Pubmed:10820186]
J Neurochem. 2000 Jun;74(6):2268-77.
An excess of the free radical nitric oxide (NO) is viewed as a deleterious factor involved in various CNS disorders. Numerous studies have shown that the Ginkgo biloba extract EGb 761 is a NO scavenger with neuroprotective properties. However, the mechanisms underlying its neuroprotective ability remain to be fully established. Thus, we investigated the effect of different constituents of EGb 761, i.e., flavonoids and terpenoids, against toxicity induced by NO generators on cells of the hippocampus, a brain area particularly susceptible to neurodegenerative damage. Exposure of rat primary mixed hippocampal cell cultures to either sodium nitroprusside (SNP; 100 microM) or 3-morpholinosydnonimine resulted in both a decrease in cell survival and an increase in free radical accumulation. These SNP-induced events were blocked by either EGb 761 (10-100 microg/ml) or its flavonoid fraction CP 205 (25 microg/ml), as well as by inhibitors of protein kinase C (PKC; chelerythrine) and L-type calcium channels (nitrendipine). In contrast, the terpenoid constituents of EGb 761, known as bilobalide and ginkgolide B, as well as inhibitors of phospholipases A [3-[(4-octadecyl)benzoyl]acrylic acid (OBAA)] and C (U-73122), failed to display any significant effects. Moreover, EGb 761 (50 microm) CP 205 (25 microg/ml), and chelerythrine were also able to rescue hippocampal cells preexposed to SNP (up to 1 mM). Finally, EGb 761 (100 microg/ml) was shown to block the activation of PKC induced by SNP (100 microM). These data suggest that the protective and rescuing abilities of EGb 761 are not only attributable to the antioxidant properties of its flavonoid constituents but also via their ability to inhibit NO-stimulated PKC activity.
Phospholipase A(2) is involved in thapsigargin-induced sodium influx in human lymphocytes.[Pubmed:10666300]
Arch Biochem Biophys. 2000 Feb 15;374(2):213-21.
Previously, we reported that emptying of intracellular Ca(2+) pools with endoplasmatic Ca(2+)-ATP-ase inhibitor thapsigargin leads to the Na(+) influx in human lymphocytes (M. Tepel et al., 1994, J. Biol. Chem. 269, 26239-26242). In the present study we examined the mechanism underlying the thapsigargin-induced Na(+) entry. We found that the thapsigargin-induced increase in Na(+) concentration was effectively inhibited by three structurally unrelated phospholipase A(2) (PLA(2)) inhibitors, p-bromophenacyl bromide, 3-(4-octadecyl)-benzoylacrylic acid (OBAA), and bromoenol lactone (BEL). The thapsigargin-induced Na(+) influx could be mimicked by PLA(2) exogenously added to the lymphocyte suspension. In addition, thapsigargin stimulated formation of arachidonic acid (AA), the physiological PLA(2) product. AA induced Na(+) entry in a time- and concentration-dependent fashion. Both, thapsigargin-induced Na(+) influx and AA liberation were completely inhibited in the presence of tyrosine kinase inhibitor genistein but not in the absence of extracellular Ca(2+). Collectively, these data show that thapsigargin-induced Na(+) entry is associated with tyrosine kinase-dependent stimulation of PLA(2).
Beta1-integrins mediate Ca2+-signalling and T cell spreading via divergent pathways.[Pubmed:10433522]
Cell Signal. 1999 Aug;11(8):611-9.
Interaction of Jurkat T-lymphocytes with two extracellular matrix (ECM) proteins of the basement membrane, laminin or collagen type IV, combined with poly-L-lysine resulted in a strong adhesion, a highly increased intracellular Ca2+-concentration ([Ca2]i), as compared to cells on laminin or collagen type IV alone and in spreading of the cells. The strong adhesion was independent of an increase in [Ca2+]i, was not mediated by a beta1-integrin, and was due to charge interaction between the positively charged polyaminoacid and the negatively charged cell surface. The latter was confirmed by substitution of poly-L-lysine by other positively charged polyaminoacids. In contrast, Ca+-signalling and spreading of the cells adhering to laminin or collagen type IV combined with poly-L-lysine was completely blocked by anti-beta1 mAb. However, spreading of the cells was independent of an increase in [Ca2+]i suggesting divergent signal transduction pathways leading to Ca2+-signalling and spreading of the cells. We elucidated these signal transduction pathways by inhibition of key enzymes involved. The tyrosine kinase inhibitor genistein blocked Ca2+-signalling as well as spreading, whereas inhibitors of PKC (calphostin C, GF109203x), PLCgamma (U73122) and PLA2 (bromophenacyl-bromide (BPB), 3-[4-octadecyl)benzoyl]acrylic acid (OBAA)) selectively blocked spreading of the cells.
Calcium entry in Trypanosoma brucei is regulated by phospholipase A2 and arachidonic acid.[Pubmed:9841878]
Biochem J. 1998 Dec 15;336 ( Pt 3):659-66.
In contrast with mammalian cells, little is known about the control of Ca2+ entry into primitive protozoans. Here we report that Ca2+ influx in pathogenic Trypanosoma brucei can be regulated by phospholipase A2 (PLA2) and the subsequent release of arachidonic acid (AA). Several PLA2 inhibitors blocked Ca2+ entry; 3-(4-octadecyl)-benzoylacrylic acid (OBAA; IC50 0.4+/-0.1 microM) was the most potent. We identified in live trypanosomes PLA2 activity that was sensitive to OBAA and could be stimulated by Ca2+, suggesting the presence of positive feedback control. The cell-associated PLA2 activity was able to release [14C]AA from labelled phospholipid substrates. Exogenous AA (5-50 microM) also initiated Ca2+ entry in a manner that was inhibited by the Ca2+ antagonist La3+ (100 microM). Ca2+ entry did not depend on AA metabolism or protein kinase activation. The cell response was specific for AA, and fatty acids with greater saturation than tetraeicosanoic acid (AA) or with chain lengths less than C20 exhibited greatly diminished ability to initiate Ca2+ influx. Myristate and palmitate inhibited PLA2 activity and also inhibited Ca2+ influx. Overall, these results demonstrate that Ca2+ entry into T. brucei can result from phospholipid hydrolysis and the release of eicosanoic acids.
Phospholipase A2 is not responsible for lysophosphatidylcholine-induced damage in cardiomyocytes.[Pubmed:9815086]
Am J Physiol. 1998 Nov;275(5):H1782-7.
Lysophosphatidylcholine (LPC) is known to increase the intracellular concentration of Ca2+ ([Ca2+]i), leading to cell damage. In the present study we examined whether LPC activates phospholipase A2 (PLA2) and whether the activation of PLA2 is responsible for the LPC-induced cell damage in isolated rat cardiomyocytes. LPC (15 microM) produced an increase in [Ca2+]i, a change in cell shape from rod to round, and the release of creatine kinase (CK) accompanied by a significant elevation of the cellular level of nonesterified fatty acids (NEFA), especially arachidonic acid. Three PLA2 inhibitors, 7, 7-dimethyl-(5Z,8Z)-eicosadienoic acid (DEDA), 3-(4-octadecylbenzoyl)acrylic acid (OBAA), and manoalide, attenuated the LPC-induced accumulation of unsaturated NEFA to a similar degree. Nevertheless, whereas both DEDA and OBAA attenuated the LPC-induced increase in [Ca2+]i, change in cell shape, and release of CK, manoalide attenuated none of them. In the Ca2+-free solution, LPC did not increase [Ca2+]i with significantly less accumulation of NEFA, but it changed the cell shape from rod to round and increased the release of CK. These results suggest that exogenous LPC increases the PLA2 activity, which, however, may not be responsible for the LPC-induced damage in cardiomyocytes.
Inhibitors of phospholipase promote apoptosis of human endothelial cells.[Pubmed:9133633]
J Biochem. 1997 Mar;121(3):612-8.
In order to understand the signal transduction system that regulates apoptosis of human umbilical vein endothelial cells (HUVEC), we investigated the effects of inhibitors of the activity of phospholipases. All three tested inhibitors of phospholipase A2 (PLA2), namely, manoalide, 3-(4-octadecyl)benzoylacrylic acid (OBAA), and oleyoxyethylphosphorylcholine (OOPC), induced apoptotic cell death of HUVEC. After 16 h of treatment, almost all of the cells had disintegrated into apoptotic bodies, and DNA ladders characteristic of apoptotic cell death were clearly observed upon analysis of DNA on agarose gels. The release of arachidonic acid from the cells that had been treated with manoalide, OBAA or OOPC (at the same concentrations as those at which these compounds induced apoptosis) was inhibited. We also studied the effects of two inhibitors of phosphatidylinositol-specific phospholipase C (PLC), U73122, and compound 48/80. Both compounds promoted the apoptosis of HUVEC. After 16 h of treatment, few cells remained intact, and DNA fragmentation was clearly detectable after only 12 h. Quantitation of inositol released from cells treated with U73122 and compound 48/80 showed that the release of inositol was blocked. By contrast, U73343, a similar aminosteroid that does not inactivate PLC, had no such effects. Our results suggest that PLA2 and phosphatidylinositol-specific PLC might be involved in the signaling pathway of apoptosis in HUVEC, and that the metabolism of arachidonic acid and of inositol might play important roles in the present apoptotic signal-transduction system.
Phospholipase A2 activation is not required for long-term synaptic depression.[Pubmed:7737325]
Eur J Pharmacol. 1995 Feb 6;273(3):R7-9.
Low-frequency synaptic stimulation evokes long-term depression of synaptic strength. One hypothesis is that modification of AMPA receptors by phospholipase A2 causes long-term depression. A previous study reported bromophenacylbromide, a completely nonselective phospholipase A2 inhibitor, blocked long-term depression at Schaffer collateral-CA1 synapses in hippocampus. In contrast, I show here that 3-(4-octadecyl)-benzoylacrylic acid (OBAA), a much more potent and selective inhibitor of low and high molecular weight phospholipase A2, does not block long-term depression at these same synapses, indicating that phospholipase A2 is not necessary for modifications causing long-term depression.
Phospholipase A2 inhibition by alkylbenzoylacrylic acids.[Pubmed:1324685]
Biochem Pharmacol. 1992 Aug 18;44(4):805-13.
3-(4-Alkylbenzoyl)acrylic acids (ABAAs) were synthesized by acylation of alkylbenzenes with maleic anhydride and then screened in vitro for inhibition of phospholipase A2 (PLA2) from snake venom and from porcine pancreas. The inhibitory potency of ABAAs increased with the length of the alkyl residues resulting in IC50 values of between 10(-7) and 10(-4) mol/L. The most potent inhibitors of the snake venom PLA2 were the 4-(n)-hexadecyl and octadecyl (OBAA) derivatives. Kinetic experiments referred to a time-dependent inhibitory reaction. Irreversibility was examined by dilution and dialysis. A molar ratio of inactivation of OBAA of nearly 20 was estimated. Double reciprocal replots of the apparent inactivation constants to the concentration of OBAA gave a (pseudo) first order rate constant of inactivation of 2.3 min-1. For the dissociation constant of the enzyme-inhibitor intermediate, a value of 6 x 10(-6) mol/L was obtained. On the other hand, the PLA2 from porcine pancreas seemed hardly to be inhibited by ABAAs. The present data are discussed in relation to the proposed model for PLA2 inactivation by manoalide. In human PMNs leukotriene B4 and 5-HETE production was essentially reduced. In human platelets the thrombin-induced TxA2 production was reduced. Since these effects disappeared after addition of arachidonic acid, these findings refer to a PLA2 inhibition. The immunologically induced bronchospasm in guinea pigs was significantly and dose-dependently inhibited by OBAA. This indicates that ABAAs might be useful in treating allergic diseases, such as asthma, eczema, allergic shock and others.


