NifedipineCa2+ channel blocker (L-type) CAS# 21829-25-4 |
- Sulfo-NHS-Biotin
Catalog No.:BCC3576
CAS No.:119616-38-5
- Sulfo-NHS-LC-Biotin
Catalog No.:BCC3578
CAS No.:127062-22-0
- NHS-Biotin
Catalog No.:BCC3577
CAS No.:35013-72-0
- Biotin Hydrazide
Catalog No.:BCC3582
CAS No.:66640-86-6
- NHS-LC-Biotin
Catalog No.:BCC3579
CAS No.:72040-63-2
- Iodoacetyl-LC-Biotin
Catalog No.:BCC3584
CAS No.:93285-75-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 21829-25-4 | SDF | Download SDF |
| PubChem ID | 4485 | Appearance | Powder |
| Formula | C17H18N2O6 | M.Wt | 346.33 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (288.74 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
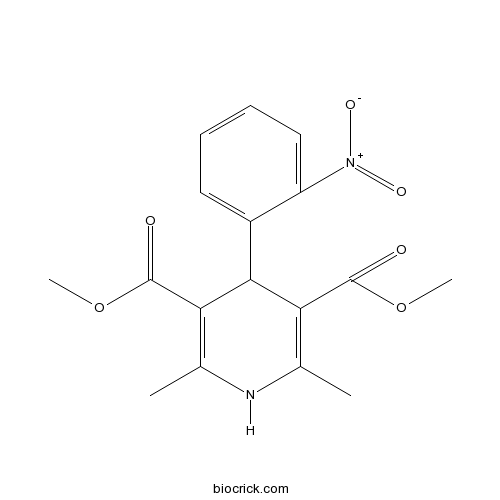
| Chemical Name | dimethyl 2,6-dimethyl-4-(2-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate | ||
| SMILES | CC1=C(C(C(=C(N1)C)C(=O)OC)C2=CC=CC=C2[N+](=O)[O-])C(=O)OC | ||
| Standard InChIKey | HYIMSNHJOBLJNT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H18N2O6/c1-9-13(16(20)24-3)15(14(10(2)18-9)17(21)25-4)11-7-5-6-8-12(11)19(22)23/h5-8,15,18H,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | L-type calcium channel blocker. |

Nifedipine Dilution Calculator

Nifedipine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8874 mL | 14.4371 mL | 28.8742 mL | 57.7484 mL | 72.1855 mL |
| 5 mM | 0.5775 mL | 2.8874 mL | 5.7748 mL | 11.5497 mL | 14.4371 mL |
| 10 mM | 0.2887 mL | 1.4437 mL | 2.8874 mL | 5.7748 mL | 7.2185 mL |
| 50 mM | 0.0577 mL | 0.2887 mL | 0.5775 mL | 1.155 mL | 1.4437 mL |
| 100 mM | 0.0289 mL | 0.1444 mL | 0.2887 mL | 0.5775 mL | 0.7219 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Nifedipine
- Vindoline
Catalog No.:BCN4933
CAS No.:2182-14-1
- SRPIN340
Catalog No.:BCC4849
CAS No.:218156-96-8
- SC 26196
Catalog No.:BCC7880
CAS No.:218136-59-5
- Isopteleine
Catalog No.:BCN7067
CAS No.:2181-84-2
- Potassium Canrenoate
Catalog No.:BCC3844
CAS No.:2181-04-6
- Thalifoline
Catalog No.:BCN3301
CAS No.:21796-15-6
- Noroxyhydrastinine
Catalog No.:BCN2646
CAS No.:21796-14-5
- Homomangiferin
Catalog No.:BCN8145
CAS No.:21794-66-1
- Diallyl disulfide
Catalog No.:BCN3840
CAS No.:2179-57-9
- Curcolonol
Catalog No.:BCN3558
CAS No.:217817-09-9
- 9,16-Dioxo-10,12,14-octadecatrienoic acid
Catalog No.:BCN1490
CAS No.:217810-46-3
- H-Asp(OBzl)-OH
Catalog No.:BCC2885
CAS No.:2177-63-1
- Macrocarpal K
Catalog No.:BCN4934
CAS No.:218290-59-6
- BDNF (human)
Catalog No.:BCC5944
CAS No.:218441-99-7
- Octahydroisoindole
Catalog No.:BCN2275
CAS No.:21850-12-4
- Falcarinol
Catalog No.:BCN3938
CAS No.:21852-80-2
- Bardoxolone
Catalog No.:BCC1399
CAS No.:218600-44-3
- Bardoxolone methyl
Catalog No.:BCC1400
CAS No.:218600-53-4
- Boc- ß-HoIle-OH
Catalog No.:BCC3236
CAS No.:218608-82-3
- 12-Hydroxyisodrimenin
Catalog No.:BCN4935
CAS No.:218780-16-6
- Boc-Arg(NO2)-OH
Catalog No.:BCC3065
CAS No.:2188-18-3
- Lycorine chloride
Catalog No.:BCN1220
CAS No.:2188-68-3
- Horminone
Catalog No.:BCN4936
CAS No.:21887-01-4
- Boc-Orn-OH
Catalog No.:BCC3427
CAS No.:21887-64-9
Attenuation of Cardiovascular Response to Direct Laryngoscopy and Intubation, Comparative Study of Lignocaine, Nifedipine, and Placebo During General Anesthesia.[Pubmed:28298755]
Anesth Essays Res. 2017 Jan-Mar;11(1):47-51.
BACKGROUND/OBJECTIVE: The purpose of the study was to compare the attenuation of cardiovascular response to direct laryngoscopy and intubation using lignocaine, Nifedipine, and placebo during general anesthesia. MATERIALS AND METHODS: This prospective study was done in sixty patients undergoing noncardiac surgeries of American Society of Anesthesiologists health status Class I and II between the age groups of 18-60 years. They were randomly divided into three groups of 20 each (lignocaine group, Nifedipine group, and placebo group) and cardiovascular response (heart rate [HR] and blood pressure [BP]) to direct laryngoscopy and intubation were compared. RESULTS: The rise in HR and BP was most significant in the placebo group and insignificant in lignocaine and Nifedipine groups. CONCLUSION: Nifedipine is effective than lignocaine in attenuating hypertensive response, and lignocaine is effective in attenuating rate pressure product than Nifedipine.
Nifedipine Increases Iron Content in WKPT-0293 Cl.2 Cells via Up-Regulating Iron Influx Proteins.[Pubmed:28243203]
Front Pharmacol. 2017 Feb 13;8:60.
Nifedipine was reported to enhance urinary iron excretion in iron overloaded mice. However, it remains unknown how Nifedipine stimulates urinary iron excretion in the kidney. We speculated that Nifedipine might inhibit the TfR1/ DMT1 (transferrin receptor 1/divalent metal transporter1)-mediated iron uptake by proximal tubule cells in addition to blocking L-type Ca2+ channels, leading to an increase in iron in lumen-fluid and then urinary iron excretion. To test this hypothesis, we investigated the effects of Nifedipine on iron content and expression of TfR1, DMT1 and ferroportin1 (Fpn1) in WKPT-0293 Cl.2 cells of the S1 segment of the proximal tubule in rats, using a graphite furnace atomic absorption spectrophotometer and Western blot analysis, respectively. We demonstrated for the first time that Nifedipine significantly enhanced iron content as well as TfR1 and DMT1 expression and had no effect on Fpn1 levels in the cells. We also found that ferric ammonium citrate decreased TfR1 levels, increased Fpn1 expression and had no effect on DMT1 content, while co-treatment with Nifedipine and FAC increase TfR1 and DMT1 expression and also had no effect on Fpn1 levels. These findings suggest that the Nifedipine-induced increase in cell iron may mainly be due to the corresponding increase in TfR1 and DMT1 expression and also imply that the effects of Nifedipine on iron transport in proximal tubule cells can not explain the increase in urinary iron excretion.
Effect of azithromycin on gingival overgrowth induced by cyclosporine A + nifedipine combination therapy: A morphometric analysis in rats.[Pubmed:28298821]
J Indian Soc Periodontol. 2016 Jul-Aug;20(4):396-401.
BACKGROUND: Drug-induced gingival overgrowth (DIGO) is a well-known adverse effect of cyclosporine A (CsA) and Nifedipine (Nf) therapy. The aim of the present morphometric study was to evaluate the effect of azithromycin (Azi) on the combined GO in rats induced by CsA + Nf combination. MATERIALS AND METHODS: Thirty Sprague-Dawley male rats were randomly divided equally into three groups. Group 1 (control) received olive oil only; Group 2 received a combination of CsA and Nf in olive oil throughout the study period; Group 3 received CsA + Nf combination therapy, and Azi was added for 1 week in the 5(th) week. All the drugs were delivered by oral route. Impressions of the mandibular central incisal regions were taken, and study models were prepared at baseline and biweekly up to the 8 weeks. Statistical analysis was done by one-way analysis of variance and intergroup comparisons were made using Tukey's post hoc analysis. RESULTS: Significant GO was evident in Group 2 and Group 3 rats when compared to Group 1. However, in Group 3 (Azi), GO was observed up to the 4(th) week, but a significant decrease in GO was noticed during 6-8(th) week after the administration of Azi in 5(th) week. CONCLUSION: Azi is an effective drug in the remission of DIGO induced by combined therapy of CsA + Nf and thereby can be considered as a useful therapeutic regimen in minimizing the DIGO in transplant patients.
Maintenance nifedipine therapy for preterm symptomatic placenta previa: A randomized, multicenter, double-blind, placebo-controlled trial.[Pubmed:28333939]
PLoS One. 2017 Mar 23;12(3):e0173717.
OBJECTIVE: To assess the impact of maintenance Nifedipine therapy on pregnancy duration in women with preterm placenta previa bleeding. METHODS: PPADAL was a randomized, double-blind, placebo-controlled trial conducted between 05/2008 and 05/2012 in five French hospitals. The trial included 109 women, aged >/= 18 years, with at least one episode of placenta previa bleeding, intact membranes and no other pregnancy complication, at gestational age 24 to 34 weeks and after 48 hours of complete acute tocolysis. Women were randomly allocated to receive either 20 mg of slow-release Nifedipine three times daily (n = 54) or placebo (n = 55) until 36 + 6 weeks of gestation. The primary outcome for the trial was length of pregnancy measured in days after enrolment. Main secondary outcomes were rates of recurrent bleeding, cesarean delivery due to hemorrhage, blood transfusion, maternal side effects, gestational age at delivery and adverse perinatal outcomes (perinatal death, chronic lung disease, neonatal sepsis, intraventricular hemorrhage > grade 2, perventricular leukomalacia > grade 1, or necrotizing enterocolitis). Analysis was by intention to treat. RESULTS: Mean (SD) prolongation of pregnancy was not different between the Nifedipine (n = 54) and the placebo (n = 55) group; 42.5 days +/- 23.8 versus 44.2 days +/- 24.5, p = 0.70. Cesarean due to hemorrhage performed before 37 weeks occurred more frequently in the Nifedipine group in comparison with the placebo group (RR, 1.66; 95% confidence interval, 1.05-2.72). Adverse perinatal outcomes were comparable between groups; 3.8% for Nifedipine versus 5.5% for placebo (relative risk, 0.52; 95% confidence interval 0.10-2.61). No maternal mortality or perinatal death occurred. CONCLUSION: Maintenance oral Nifedipine neither prolongs duration of pregnancy nor improves maternal or perinatal outcomes. TRIAL REGISTRATION: ClinicalTrials.gov NCT00620724.
Pharmacological reactivity of human epicardial coronary arteries: phasic and tonic responses to vasoconstrictor agents differentiated by nifedipine.[Pubmed:7889259]
Br J Pharmacol. 1994 Dec;113(4):1093-8.
1. Human epicardial coronary artery rings, freshly obtained from cardiac transplantation patients, commonly exhibited phasic contractile activity in vitro. This activity occurred either spontaneously or in response to vasoconstrictor stimulation. 2. Nifedipine pretreatment (1 nM-0.1 microM) reduced both types of phasic contractions in a concentration-dependent manner. At 0.1 microM Nifedipine, spontaneous contractions were completely abolished, as were phasic contractions induced by U46619, endothelin-1 or 5-hydroxytryptamine (5-HT). 3. For U46619 (0.1-100 nM), the largest phasic contractions (amplitude peak to trough) occurred over the mid-range of concentrations used (1-10 nM). At higher concentrations (30-100 nM), phasic activity was reduced as the response reached a maximum. Estimated pEC50 values for the upper phasic and lower phasic curves were significantly different (8.71 +/- 0.13 versus 7.90 +/- 0.11; P < 0.05; n = 10). In the presence of nifidepine (0.1 microM), the purely tonic contraction curve to U46619 was similar to the lower phasic curve in the absence of Nifedipine (pEC50 = 8.14 +/- 0.06, n = 10). Similar results were obtained for endothelin-1 (0.1-100 nM). 4. Responses to 5-HT (1 nM-3 microM) were more variable. The largest phasic contractions were spread unevenly throughout the concentration-response curve. In the presence of Nifedipine (0.1 microM), the curve to 5-HT was significantly depressed in range but not sensitivity (pEC50) when compared with the phasic curves. 5. In conclusion, activation of dihydropyridine-sensitive voltage-operated Ca2+ channels mediated the phasic contractions commonly observed in human epicardial coronary arteries. These contractions amplified the contractile responses to low concentrations of vasoconstrictors. Inhibition of phasic activity by the Ca2+ channel antagonist, Nifedipine, allowed the tonic vasoconstrictor profile of human isolated coronary artery to be determined which is important information for the accurate quantitative assessment of vasodilator responses in this tissue in vitro.


