Bardoxolone methylIKK inhibitor, potent antioxidant inflammation modulator CAS# 218600-53-4 |
- MLN8237 (Alisertib)
Catalog No.:BCC2166
CAS No.:1028486-01-2
- VX-680 (MK-0457,Tozasertib)
Catalog No.:BCC2167
CAS No.:639089-54-6
- MK-8745
Catalog No.:BCC3994
CAS No.:885325-71-3
- AT9283
Catalog No.:BCC2173
CAS No.:896466-04-9
- GSK1070916
Catalog No.:BCC2183
CAS No.:942918-07-2
- CCT129202
Catalog No.:BCC2187
CAS No.:942947-93-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 218600-53-4 | SDF | Download SDF |
| PubChem ID | 400769 | Appearance | Powder |
| Formula | C32H43NO4 | M.Wt | 505.69 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | NSC 713200; RTA 402; CDDO Methyl ester | ||
| Solubility | DMSO : 25 mg/mL (49.44 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
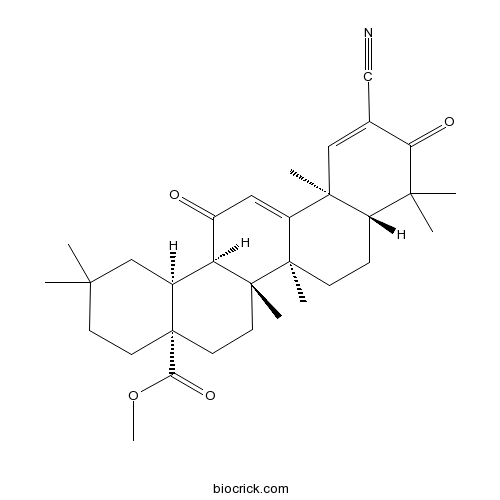
| Chemical Name | methyl (4aS,6aR,6bS,8aR,12aS,14aR,14bS)-11-cyano-2,2,6a,6b,9,9,12a-heptamethyl-10,14-dioxo-1,3,4,5,6,7,8,8a,14a,14b-decahydropicene-4a-carboxylate | ||
| SMILES | CC1(CCC2(CCC3(C(C2C1)C(=O)C=C4C3(CCC5C4(C=C(C(=O)C5(C)C)C#N)C)C)C)C(=O)OC)C | ||
| Standard InChIKey | WPTTVJLTNAWYAO-KPOXMGGZSA-N | ||
| Standard InChI | InChI=1S/C32H43NO4/c1-27(2)11-13-32(26(36)37-8)14-12-31(7)24(20(32)17-27)21(34)15-23-29(5)16-19(18-33)25(35)28(3,4)22(29)9-10-30(23,31)6/h15-16,20,22,24H,9-14,17H2,1-8H3/t20-,22-,24-,29-,30+,31+,32-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Bardoxolone methyl is an oral modulator of antioxidant inflammation. | |||||
| Targets | antioxidant inflammation | |||||

Bardoxolone methyl Dilution Calculator

Bardoxolone methyl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9775 mL | 9.8875 mL | 19.775 mL | 39.5499 mL | 49.4374 mL |
| 5 mM | 0.3955 mL | 1.9775 mL | 3.955 mL | 7.91 mL | 9.8875 mL |
| 10 mM | 0.1977 mL | 0.9887 mL | 1.9775 mL | 3.955 mL | 4.9437 mL |
| 50 mM | 0.0395 mL | 0.1977 mL | 0.3955 mL | 0.791 mL | 0.9887 mL |
| 100 mM | 0.0198 mL | 0.0989 mL | 0.1977 mL | 0.3955 mL | 0.4944 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Bardoxolone methyl is an activator of the KEAP1-Nrf2 pathway [1] and also inhibits the pro-inflammatory transcription factor NF-kB [2] which can protect kidneys from aristolochic acid (AA)-induced acute kidney injury (AKI) with IC50 value of 1.5 nM and LC50 value of 2.1 µM [3].
Nrf2, a transcription factor, is a basic leucine zipper (bZIP) protein that regulates the expression of antioxidant proteins that protect against oxidative damage triggered by injury and inflammation [4], such as NADPH, Glutathione, SRXN1, TXNRD1, HMOX1, GST, UGT and Mrps. Nrf2 plays an important role in the maintenance of homeostasis which can control the basal and inducible expression of a battery of genes with diverse physiological roles, including the preservation of redox balance, the metabolism and detoxification of xenobiotics, and the regulation of multiple metabolic pathways that ensure the provision of cellular energy[5].
Bardoxolone methyl is a synthetic oleanane triterpenoid compound, which has no effect on the function and histology of normal kidneys but increased renal expression of Nrf2, HO-1 and NQO1 by western blotting analysis of mice kidneys and immunofluorescence staining, and can prevent AA-induced acute kidney injury and reduce AAI-induced TI injury in mRNA and protein levels through real-time PCR.[6] In conclusion, Bardoxolone methyl can prevent AAI-induced renal damage, and it may exert this renoprotective effects by activating the Nrf2 signaling pathway and inducing the downstream target genes expression. A phase 3 clinical trial evaluating bardoxolone methyl for the treatment of chronic kidney disease (CKD) was terminated in October 2012 after patients treated with the drug were found to have experienced a higher rate of heart-related adverse events, including heart failure, hospitalizations and deaths.[7] Now in 2014, Kyowa Hakko Kirin announced plans to evaluate both safety and efficacy of bardoxolone methyl in a Phase 2 clinical study to be performed in Japan for the treatment of CKD associated with type 2 diabetes.[8]
References:
1.Yates MS, Tauchi M, Katsuoka F, et al."Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2-regulated genes." Mol Cancer Ther 2007, 6 (1): 154–62.
2.Ahamd R, Raina D, Meyer C, et al.. "Triterpenoid CDDO-Me blocks the NF-kappaB pathway by direct inhibition of IKKbeta on Cys-179.". J Biol Chem, 2006, 281 (47): 35764–9.
3.Ian M. Copple. et al. Chemical Tuning Enhances Both Potency Toward Nrf2 and In Vitro Therapeutic Index of Triterpenoids. TOXICOLOGICAL SCIENCES,2014,140(2), 462–469.
4.Gold R, Kappos L. Et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N. Engl. J. Med. 2012, 367 (12): 1098–107.
5.Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013,53:401–426.
6.Juan Wua. et al. Bardoxolone methyl (BARD) ameliorates aristolochic acid (AA)-induced acute kidney injury through Nrf2 pathway. Toxicology. 2014, 318(6):22–31.
7.de Zeeuw D, Akizawa T, Audhya P, et al. "Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease.". N Engl J Med,2013,369 (26): 2492–503.
8.Kyowa Hakko Kirin Co Ltd announces future development direction for bardoxolone methyl (RTA 402).
- Bardoxolone
Catalog No.:BCC1399
CAS No.:218600-44-3
- Falcarinol
Catalog No.:BCN3938
CAS No.:21852-80-2
- Octahydroisoindole
Catalog No.:BCN2275
CAS No.:21850-12-4
- BDNF (human)
Catalog No.:BCC5944
CAS No.:218441-99-7
- Macrocarpal K
Catalog No.:BCN4934
CAS No.:218290-59-6
- Nifedipine
Catalog No.:BCC4808
CAS No.:21829-25-4
- Vindoline
Catalog No.:BCN4933
CAS No.:2182-14-1
- SRPIN340
Catalog No.:BCC4849
CAS No.:218156-96-8
- SC 26196
Catalog No.:BCC7880
CAS No.:218136-59-5
- Isopteleine
Catalog No.:BCN7067
CAS No.:2181-84-2
- Potassium Canrenoate
Catalog No.:BCC3844
CAS No.:2181-04-6
- Thalifoline
Catalog No.:BCN3301
CAS No.:21796-15-6
- Boc- ß-HoIle-OH
Catalog No.:BCC3236
CAS No.:218608-82-3
- 12-Hydroxyisodrimenin
Catalog No.:BCN4935
CAS No.:218780-16-6
- Boc-Arg(NO2)-OH
Catalog No.:BCC3065
CAS No.:2188-18-3
- Lycorine chloride
Catalog No.:BCN1220
CAS No.:2188-68-3
- Horminone
Catalog No.:BCN4936
CAS No.:21887-01-4
- Boc-Orn-OH
Catalog No.:BCC3427
CAS No.:21887-64-9
- 1-Phenyloctane
Catalog No.:BCN2227
CAS No.:2189-60-8
- Taraxeryl acetate
Catalog No.:BCN4937
CAS No.:2189-80-2
- Ciwujiatone
Catalog No.:BCN7598
CAS No.:218901-26-9
- Euphorbia factor L2
Catalog No.:BCN3783
CAS No.:218916-51-9
- 5,15-Diacetyl-3-benzoyllathyrol
Catalog No.:BCN1196
CAS No.:218916-52-0
- Euphorbia factor L8
Catalog No.:BCN3785
CAS No.:218916-53-1
Bardoxolone methyl modulates efflux transporter and detoxifying enzyme expression in cisplatin-induced kidney cell injury.[Pubmed:27480280]
Toxicol Lett. 2016 Sep 30;259:52-59.
Cisplatin is prescribed for the treatment of solid tumors and elicits toxicity to kidney tubules, which limits its clinical use. Nuclear factor erythroid 2-related factor 2 (Nrf2, NFE2L2) is a critical transcription factor that has been shown to protect against kidney injury through activation of antioxidant mechanisms. We aimed to evaluate the ability of short-term treatment with the Nrf2 activator Bardoxolone methyl (CDDO-Me) to protect against cisplatin-induced kidney cell toxicity. Cell viability was assessed in human kidney proximal tubule epithelial cells (hPTCs) exposed to low, intermediate, and high cisplatin concentrations in the presence and absence of CDDO-Me, administered either prior to or after cisplatin. Treatment with cisplatin alone resulted in reductions in hPTC viability, while CDDO-Me administered prior to or after cisplatin exposure yielded significantly higher cell viability (17%-71%). Gene regulation (mRNA expression) studies revealed the ability of CDDO-Me to modify protective pathways including Nrf2 induced detoxifying genes [GCLC (increased 1.9-fold), NQO1 (increased 9.3-fold)], and an efflux transporter [SLC47A1 (increased 4.5-fold)] at 12h. Protein assessments were in agreement with gene expression. Immunofluorescence revealed localization of GCLC and NQO1 to the nucleus and cytosol, respectively, with CDDO-Me administered prior to or after cisplatin exposure. The findings of enhanced cell viability and increased expression of detoxifying enzymes (GCLC and NQO1) and the multidrug and toxin extrusion protein 1 (MATE1) efflux transporter (SLC47A1) in hPTCs exposed to CDDO-Me, suggest that intermittent treatment with CDDO-Me prior to or after cisplatin exposure may be a promising approach to mitigate acute kidney injury.
Bardoxolone Methyl and a Related Triterpenoid Downregulate cMyc Expression in Leukemia Cells.[Pubmed:28275049]
Mol Pharmacol. 2017 May;91(5):438-450.
Structurally related pentacyclic triterpenoids methyl 2-cyano-3,12-dioxoolean-1,9-dien-28-oate [bardoxolone-methyl (Bar-Me)] and methyl 2-trifluoromethyl-3,11-dioxoolean-1,12-dien-30-oate (CF3DODA-Me) contain 2-cyano-1-en-3-one and 2-trifluoromethyl-1-en-3-one moieties, respectively, in their A-rings and differ in the position of their en-one structures in ring C. Only Bar-Me forms a Michael addition adduct with glutathione (GSH) and inhibits IKKbeta phosphorylation. These differences may be due to steric hindrance by the 11-keto group in CF3DODA-Me, which prevents Michael addition by the conjugated en-one in the A-ring. In contrast, both Bar-Me and CF3DODA-Me induce reactive oxygen species in HL-60 and Jurkat leukemia cells, inhibit cell growth, induce apoptosis and differentiation, and decrease expression of specificity proteins (Sp) 1, 3, and 4, and cMyc, and these effects are significantly attenuated after cotreatment with the antioxidant GSH. In contrast to solid tumor-derived cells, cMyc and Sp transcriptions are regulated independently and cMyc plays a more predominant role than Sp transcription factors in regulating HL-60 or Jurkat cell proliferation and differentiation compared with that observed in cells derived from solid tumors.
Bardoxolone-methyl inhibits migration and metabolism in MCF7 cells.[Pubmed:28277986]
Free Radic Res. 2017 Feb;51(2):211-221.
Bardoxolone-methyl (BAR) is reported to have anti-inflammatory, anti-proliferative and anti-fibrotic effects. BAR activates Nrf2 and may ameliorate oxidative stress through induction of antioxidant genes. However, off-target effects, probably concentration and NFkB-dependent, have limited the clinical use of BAR. Nrf2 regulates expression of antioxidant and mitochondrial genes and has been proposed as a target for both obesity and breast cancer. Therefore, we explored whether BAR can alter migration and proliferation in the MCF7 cell line and whether metabolic function is affected by BAR. Incubation with BAR caused a time-dependent migratory inhibition and an associated decrease in mitochondrial respiration. Both migratory and mitochondrial inhibition by BAR were further enhanced in the presence of fatty acids. In addition to the activation of Nrf2, BAR altered the expression of target mRNA GCLC and UCP1. After 24 h, BAR inhibited both glycolytic capacity, reserve (p < 0.05) and oxidative phosphorylation (p < 0.001) with an associated increase in mitochondrial ROS and loss of intracellular glutathione in MCF7 cells; however, impairment of mitochondrial activity was prevented by N-acetyl cysteine. The fatty acid, palmitate, increased mitochondrial ROS, impaired migration and oxidative phosphorylation but palmitate toxicity towards MCF7 could not be inhibited by N-acetyl cysteine suggesting that they exert effects through different pathways. BAR-activated AKT, induced DNA damage and inhibited cell proliferation. When the proteasome was inhibited, there was loss of BAR-mediated changes in p65 phosphorylation and SOD2 expression suggesting non-canonical NFkB signaling effects. These data suggest that BAR-induced ROS are important in inhibiting MCF7 migration and metabolism by negatively affecting glycolytic capacity and mitochondrial function.
Bardoxolone methyl prevents obesity and hypothalamic dysfunction.[Pubmed:27417254]
Chem Biol Interact. 2016 Aug 25;256:178-87.
High-fat (HF) diet-induced obesity is associated with hypothalamic leptin resistance and low grade chronic inflammation, which largely impairs the neuroregulation of negative energy balance. Neuroregulation of negative energy balance is largely controlled by the mediobasal and paraventricular nuclei regions of the hypothalamus via leptin signal transduction. Recently, a derivative of oleanolic acid, Bardoxolone methyl (BM), has been shown to have anti-inflammatory effects. We tested the hypothesis that BM would prevent HF diet-induced obesity, hypothalamic leptin resistance, and inflammation in mice fed a HF diet. Oral administration of BM via drinking water (10 mg/kg daily) for 21 weeks significantly prevented an increase in body weight, energy intake, hyperleptinemia, and peripheral fat accumulation in mice fed a HF diet. Furthermore, BM treatment prevented HF diet-induced decreases in the anorexigenic effects of peripheral leptin administration. In the mediobasal and paraventricular nuclei regions of the hypothalamus, BM administration prevented HF diet-induced impairments of the downstream protein kinase b (Akt) pathway of hypothalamic leptin signalling. BM treatment also prevented an increase in inflammatory cytokines, tumour necrosis factor alpha (TNFalpha) and interleukin 6 (IL-6) in these two hypothalamic regions. These results identify a potential novel neuropharmacological application for BM in preventing HF diet-induced obesity, hypothalamic leptin resistance, and inflammation.


