NevadensinCAS# 10176-66-6 |
- Lysionotin
Catalog No.:BCX0898
CAS No.:152743-19-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 10176-66-6 | SDF | Download SDF |
| PubChem ID | 160921 | Appearance | Yellow powder |
| Formula | C18H16O7 | M.Wt | 344.32 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Lysionotin;152743-19-6 | ||
| Solubility | Soluble in methan | ||
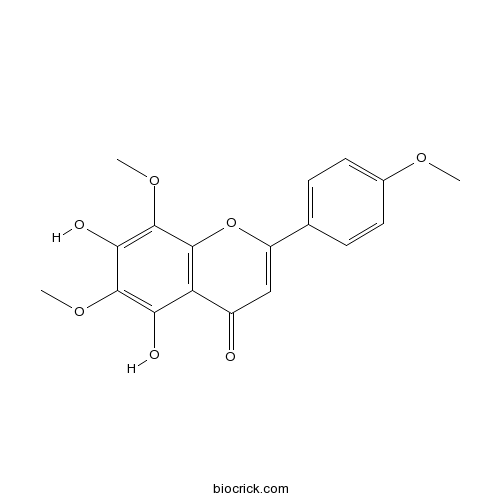
| Chemical Name | 5,7-dihydroxy-6,8-dimethoxy-2-(4-methoxyphenyl)chromen-4-one | ||
| SMILES | COC1=CC=C(C=C1)C2=CC(=O)C3=C(O2)C(=C(C(=C3O)OC)O)OC | ||
| Standard InChIKey | KRFBMPVGAYGGJE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H16O7/c1-22-10-6-4-9(5-7-10)12-8-11(19)13-14(20)17(23-2)15(21)18(24-3)16(13)25-12/h4-8,20-21H,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Nevadensin is an important herb-based constituent inhibiting estragole bioactivation. 2. Nevadensin protects against a methyleugenol-induced marker of hepatocarcinogenicity in male F344 rat. 3. Nevadensin exhibits inhibition activity against Mycobacterium tuberculosis, with equal MIC value of 200 microg/mL. 4.Lysionotin is a natural flavonoid predominantly found in fewflower lysionotus herbs and possesses many pharmacological properties, such as antibacterial, anti-inflammatory, antihypertensive, and free radical scavenging activities. It is an efficient inhibitor of α-toxin expression and shows significant protection against S. aureus in vitro and in vivo. |
| Targets | Antifection |
| In vitro | Lysionotin attenuates Staphylococcus aureus pathogenicity by inhibiting α-toxin expression.[Pubmed: 28710557 ]Appl Microbiol Biotechnol. 2017 Sep;101(17):6697-6703.α-Toxin, one of the best known pore-forming proteins produced by Staphylococcus aureus (S. aureus), is a critical virulence factor in multiple infections. The necessity of α-toxin for S. aureus pathogenicity suggests that this toxin is an important target for the development of a potential treatment strategy.
|
| Cell Research | Lysionotin attenuates Staphylococcus aureus pathogenicity by inhibiting α-toxin expression.[Pubmed: 28710557 ]Appl Microbiol Biotechnol. 2017 Sep;101(17):6697-6703.Cell lines:A549 human lung epithelial cells |
| Animal Research | Lysionotin attenuates Staphylococcus aureus pathogenicity by inhibiting α-toxin expression.[Pubmed: 28710557 ]Appl Microbiol Biotechnol. 2017 Sep;101(17):6697-6703.Animal Models: 6- to 8-week-old female C57BL/6J mice |
| Structure Identification | Acta Pharmacol Sin. 2002 Jul;23(7):667-72.Structure-activity relationship of natural flavonoids in hydroxyl radical-scavenging effects.[Pubmed: 12100765]To study the relationship between the structure and hydroxyl radical (*OH)-scavenging activity of twelve natural flavonoids.
|

Nevadensin Dilution Calculator

Nevadensin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9043 mL | 14.5214 mL | 29.0428 mL | 58.0855 mL | 72.6069 mL |
| 5 mM | 0.5809 mL | 2.9043 mL | 5.8086 mL | 11.6171 mL | 14.5214 mL |
| 10 mM | 0.2904 mL | 1.4521 mL | 2.9043 mL | 5.8086 mL | 7.2607 mL |
| 50 mM | 0.0581 mL | 0.2904 mL | 0.5809 mL | 1.1617 mL | 1.4521 mL |
| 100 mM | 0.029 mL | 0.1452 mL | 0.2904 mL | 0.5809 mL | 0.7261 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Jaceidin
Catalog No.:BCN5832
CAS No.:10173-01-0
- Sanggenofuran B
Catalog No.:BCN7194
CAS No.:1017277-40-5
- 6alpha,16,18-Trihydroxycleroda-3,13-dien-15,16-olide
Catalog No.:BCN1640
CAS No.:1017233-48-5
- (S)-(+)-4-Benzyl-3-propionyl-2-oxazolidinone
Catalog No.:BCC8399
CAS No.:101711-78-8
- Myzodendrone
Catalog No.:BCN7257
CAS No.:101705-37-7
- 6-O-p-Hydroxybenzoylaucubin
Catalog No.:BCN5831
CAS No.:1016987-87-3
- Sulfocostunolide A
Catalog No.:BCN5830
CAS No.:1016983-51-9
- Olivil monoacetate
Catalog No.:BCN4738
CAS No.:1016974-78-9
- Barpisoflavone A
Catalog No.:BCN4739
CAS No.:101691-27-4
- Cyclo(Phe-Hpro)
Catalog No.:BCN2425
CAS No.:1016899-94-7
- Cyclo(Hpro-Leu)
Catalog No.:BCN2430
CAS No.:1016899-93-6
- Drevogenin A
Catalog No.:BCN4740
CAS No.:10163-83-4
- Ladanein
Catalog No.:BCN6670
CAS No.:10176-71-3
- LPA2 antagonist 1
Catalog No.:BCC5438
CAS No.:1017606-66-4
- PK 44 phosphate
Catalog No.:BCC2366
CAS No.:1017682-65-3
- Boc-N-Me-Ser-OH
Catalog No.:BCC2613
CAS No.:101772-29-6
- Elliotinol
Catalog No.:BCN5833
CAS No.:10178-31-1
- 7-O-Demethyl-3-isomangostin hydrate
Catalog No.:BCN7882
CAS No.:
- Desacetylmatricarin
Catalog No.:BCN7258
CAS No.:10180-88-8
- TG 100801 Hydrochloride
Catalog No.:BCC1997
CAS No.:1018069-81-2
- Butenafine HCl
Catalog No.:BCC4768
CAS No.:101827-46-7
- Diclazuril
Catalog No.:BCC8937
CAS No.:101831-37-2
- 7-Z-Trifostigmanoside I
Catalog No.:BCN7869
CAS No.:1018898-17-3
- LX-4211
Catalog No.:BCC1714
CAS No.:1018899-04-1
Identification of nevadensin as an important herb-based constituent inhibiting estragole bioactivation and physiology-based biokinetic modeling of its possible in vivo effect.[Pubmed:20226806]
Toxicol Appl Pharmacol. 2010 Jun 1;245(2):179-90.
Estragole is a natural constituent of several herbs and spices including sweet basil. In rodent bioassays, estragole induces hepatomas, an effect ascribed to estragole bioactivation to 1'-sulfooxyestragole resulting in DNA adduct formation. The present paper identifies Nevadensin as a basil constituent able to inhibit DNA adduct formation in rat hepatocytes exposed to the proximate carcinogen 1'-hydroxyestragole and Nevadensin. This inhibition occurs at the level of sulfotransferase (SULT)-mediated bioactivation of 1'-hydroxyestragole. The Ki for SULT inhibition by Nevadensin was 4 nM in male rat and human liver fractions. Furthermore, Nevadensin up to 20 microM did not inhibit 1'-hydroxyestragole detoxification by glucuronidation and oxidation. The inhibition of SULT by Nevadensin was incorporated into the recently developed physiologically based biokinetic (PBBK) rat and human models for estragole bioactivation and detoxification. The results predict that co-administration of estragole at a level inducing hepatic tumors in vivo (50mg/kg bw) with Nevadensin at a molar ratio of 0.06, representing the ratio of their occurrence in basil, results in almost 100% inhibition of the ultimate carcinogen 1'-sulfooxyestragole when assuming 100% uptake of Nevadensin. Assuming 1% uptake, inhibition would still amount to more than 83%. Altogether these data point at a Nevadensin-mediated inhibition of the formation of the ultimate carcinogenic metabolite of estragole, without reducing the capacity to detoxify 1'-hydroxyestragole via glucuronidation or oxidation. These data also point at a potential reduction of the cancer risk when estragole exposure occurs within a food matrix containing SULT inhibitors compared to what is observed upon exposure to pure estragole.
Detection of interaction between lysionotin and bovine serum albumin using spectroscopic techniques combined with molecular modeling.[Pubmed:24398555]
Mol Biol Rep. 2014 Mar;41(3):1693-702.
A combination of fluorescence, UV-Vis absorption, circular dichroism (CD), Fourier transform infrared (FT-IR) and molecular modeling approaches were employed to determine the interaction between lysionotin and bovine serum albumin (BSA) at physiological pH. The fluorescence titration suggested that the fluorescence quenching of BSA by lysionotin was a static procedure. The binding constant at 298 K was in the order of 10(5) L mol(-1), indicating that a high affinity existed between lysionotin and BSA. The thermodynamic parameters obtained at different temperatures (292, 298, 304 and 310 K) showed that the binding process was primarily driven by hydrogen bond and van der Waals forces, as the values of the enthalpy change (DeltaH degrees ) and entropy change (DeltaS degrees ) were found to be -40.81 +/- 0.08 kJ mol(-1) and -35.93 +/- 0.27 J mol(-1) K(-1), respectively. The surface hydrophobicity of BSA increased upon interaction with lysionotin. The site markers competitive experiments revealed that the binding site of lysionotin was in the sub-domain IIA (site I) of BSA. Furthermore, the molecular docking results corroborated the binding site and clarified the specific binding mode. The results of UV-Vis absorption, CD and FT-IR spectra demonstrated that the secondary structure of BSA was altered in the presence of lysionotin.
Structure-activity relationship of natural flavonoids in hydroxyl radical-scavenging effects.[Pubmed:12100765]
Acta Pharmacol Sin. 2002 Jul;23(7):667-72.
AIM: To study the relationship between the structure and hydroxyl radical (*OH)-scavenging activity of twelve natural flavonoids. METHODS: The hydroxyl radical-generating chemiluminescence system with ascorbate-CuSO4-yeast-H2O2 was used to determine the hydroxyl radical-scavenging activity of twelve natural flavonoids. RESULTS: Guercetin, heliosin, hyperoside, kaempferol, baicalin, corylifolin, lysionotin, matteucinol, corylifolinin, and genistein could effectively scavenge. OH and inhibit the chemiluminescence of the system. The IC50 values (95 % confidence limits) of the flavonoids were 12.1 (9.9-14.5) g/L, 15.8(14.0-19.2) g/L, 19.5 (16.8-27.4) g/L, 20.1 (13.6-29.0) g/L, 34.6 (28.4-43.4) g/L, 66.8 (63.2-74.4) g/L, 187 (147-235) g/L, 211 (165-284) g/L, 262 (190-346) g/L, and 708 (498-994) g/L, respectively; whereas nobilelin and corylifolin-Ac could not scavenge *OH. CONCLUSION: (1) Phenolic hydroxyls in flavonoids were the main active groups capable of scavenging *OH; (2) Hydroxyl groups in ring B and A were important *OH-scavenging active groups; (3) The ortho-dihydroxyl groups in ring A and/or B could greatly enhance the *OH-scavenging activity of the rings; (4) Comparing the IC50 values of guercetin, heliosin, hyperoside, baicalin, lysionotin, and matteucinol, it was suggested that the hydroxyl groups on 3',4' position of ring B possessed high *OH-scavenging activity and the scavenging activity of hydroxyl groups in ring B was higher than that of hydroxyl groups in ring A. The hydroxyl group or glucoside on 3 position of ring C of the above mentioned 6 flavonoids was also related to the. OH-scavenging ability. (5) The structural types of flavonoids themselves could influence their *OH-scavenging activity.
Simultaneous determination and pharmacokinetic study of three isoflavones from Trifolium pratense extract in rat plasma by LC-MS/MS.[Pubmed:24898405]
Biomed Chromatogr. 2015 Feb;29(2):210-9.
A highly selective and sensitive liquid chromatography-tandem mass spectrometry has been developed and validated for simultaneous determination of three isoflavones - ononin, formononetin and biochanin A - in rat plasma using lysionotin as internal standard (IS). The plasma samples were pretreated and extracted by liquid-liquid extraction. Chromatographic separation was accomplished on a C18 column with the column temperature of 30 degrees C and a mobile phase of methanol-0.1% formic acid (75:25, v/v). The detection was accomplished by multiple-reaction monitoring scanning with positive/negative ion-switching electrospray ionization mode. The optimized mass transition ion pairs (m/z) for quantitation were 431.3/269.1 for ononin, 267.1/252.2 for formononetin, 283.2/268.2 for biochanin A and 343.2/313.3 for IS. The total run time was 8.0 min. Full validation of the assay was implemented, including selectivity, sensitivity, linearity, precision, accuracy, recovery, matrix effect and stability. This is the first report on simultaneous determination of the three major isoflavones in rat plasma after intragastric administration of Trifolium pratense extract. The results provided a significant basis for the clinical application of this herb Trifolium pratense.
Simultaneous Determination of Seven Components from Hawthorn Leaves Flavonoids in Rat Plasma by LC-MS/MS.[Pubmed:25368407]
J Chromatogr Sci. 2015 Jul;53(6):909-14.
In this study, a simple, sensitive, and throughout liquid chromatography-tandem mass spectrometry (LC-MS/MS) method was developed for the simultaneous determination of seven flavonoid compounds, namely, rutin, vitexin-4''-O-glucoside, vitexin-2''-O-rhamnoside, hyperoside, vitexin, shanyenoside A and quercetin in rat plasma after intravenous administration of hawthorn leaves flavonoids (HLF) using lysionotin as an internal standard (IS). The target compounds were extracted using protein precipitation by methanol. The detection was achieved by LC-MS/MS in multiple reaction monitoring mode. The optimal mass transition ion pairs (m/z) for quantitation were 609.3/300.1 for rutin, 593.1/413.2 for vitexin-4''-O-glucoside, 577.3/413.2 for vitexin-2''-O-rhamnoside, 463.2/300.1 for hyperoside, 431.2/311.2 for vitexin, 407.2/245.1 for shanyenoside A, 301.1/151.1 for quercetin and 343.2/313.1 for the IS, respectively. The method was fully validated with respect to specificity, sensitivity, linearity, precision, accuracy, recovery and stability experiments. A sufficiently sensitive and selective LC-MS/MS method was first developed in this study to simultaneously evaluate the pharmacokinetics of seven flavonoids in rat plasma following intravenous administration of HLF.
Antimycobacterial and antioxidant flavones from Limnophila geoffrayi.[Pubmed:14609129]
Arch Pharm Res. 2003 Oct;26(10):816-20.
The chloroform extract of the aerial part of Limnophila geoffrayi showed antimycobacterial and antioxidant activities. Bioassay-guided fractionation has led to the isolation of the flavones Nevadensin (5,7-dihydroxy-6,8,4'-trimethoxyflavone, 1) and isothymusin (6,7-dimethoxy-5,8,4'-trihydroxyflavone, 2). Both compounds 1 and 2 exhibited inhibition activity against Mycobacterium tuberculosis, with equal MIC value of 200 microg/mL. Only compound 2 exhibited antioxidant activity against the radical scavenging ability of DPPH, with the IC50 value of 7.7 microg/mL. The crude hexane, chloroform and methanol extracts as well as the pure compounds 1 and 2 did not exhibit mutagenic activity in the Bacillus subtilis recassay.
The natural basil flavonoid nevadensin protects against a methyleugenol-induced marker of hepatocarcinogenicity in male F344 rat.[Pubmed:25218219]
Food Chem Toxicol. 2014 Dec;74:28-34.
The alkenylbenzene methyleugenol occurs naturally in a variety of spices and herbs, including basil, and their essential oils. At high dose levels methyleugenol induces hepatocarcinogenicity in rodents following bioactivation to 1'-sulfooxymethyleugenol which forms DNA adducts. This study investigated whether the inhibitory effect of the basil flavonoid Nevadensin on sulfotransferase (SULT)-mediated bioactivation of methyleugenol observed in vitro would also be reflected in a reduction of DNA adduct formation and a reduction in an early marker for liver carcinogenesis in an 8-week rat study. Co-exposure to methyleugenol and Nevadensin orally resulted in a significant inhibition of liver methyleugenol DNA adduct formation and in inhibition of hepatocellular altered foci induction, representing indicators for initiation of neoplasia. These results suggest that tumor formation could be lower in rodent bioassays when methyleugenol would be dosed in a matrix containing SULT inhibitors such as Nevadensin compared to experiments using the pure methyleugenol.


