NarirutinCAS# 14259-46-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 14259-46-2 | SDF | Download SDF |
| PubChem ID | 442431 | Appearance | White powder |
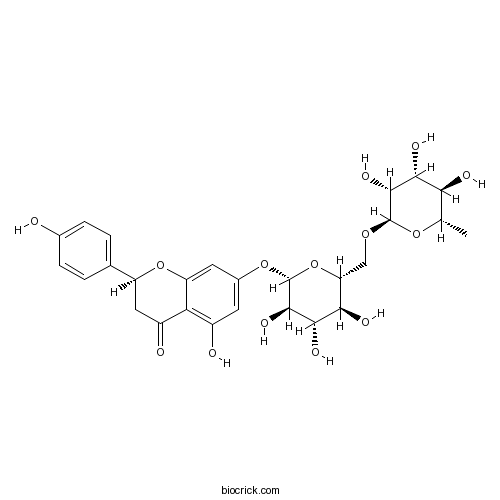
| Formula | C27H32O14 | M.Wt | 580.53 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Isonaringin; Naringenin 7-rutinoside; 4',5,7-Trihydroxyflavanone 7-rutinoside | ||
| Solubility | DMSO : 125 mg/mL (215.32 mM; Need ultrasonic) | ||
| Chemical Name | (2S)-5-hydroxy-2-(4-hydroxyphenyl)-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxan-2-yl]oxy-2,3-dihydrochromen-4-one | ||
| SMILES | CC1C(C(C(C(O1)OCC2C(C(C(C(O2)OC3=CC(=C4C(=O)CC(OC4=C3)C5=CC=C(C=C5)O)O)O)O)O)O)O)O | ||
| Standard InChIKey | HXTFHSYLYXVTHC-AJHDJQPGSA-N | ||
| Standard InChI | InChI=1S/C27H32O14/c1-10-20(31)22(33)24(35)26(38-10)37-9-18-21(32)23(34)25(36)27(41-18)39-13-6-14(29)19-15(30)8-16(40-17(19)7-13)11-2-4-12(28)5-3-11/h2-7,10,16,18,20-29,31-36H,8-9H2,1H3/t10-,16-,18+,20-,21+,22+,23-,24+,25+,26+,27+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Citrus narirutin fraction (CNF), contained 75% of narirutin, co-administration of CNF with alcohol can alleviate alcohol induced liver damage through preventing lipid formation, protecting antioxidant system and suppressing productions of pro-inflammatory cytokines. Narirutin has anti-inflammatory effect in a murine model of allergic eosinophilic airway inflammation, the mechanism is likely to be associated with a reduction in the OVA-induced increases of IL-4 and IgE. |
| Targets | NO | NOS | PGE | COX | TNF-α | NF-kB | MAPK | IL Receptor |
| In vitro | Narirutin fraction from citrus peels attenuates LPS-stimulated inflammatory response through inhibition of NF-κB and MAPKs activation.[Pubmed: 22813871]Food Chem Toxicol. 2012 Oct;50(10):3498-504.
|
| In vivo | Narirutin fraction from citrus peels attenuates alcoholic liver disease in mice.[Pubmed: 23416143]Food Chem Toxicol. 2013 May;55:637-44.
|
| Animal Research | Narirutin inhibits airway inflammation in an allergic mouse model.[Pubmed: 17600554]Clin Exp Pharmacol Physiol. 2007 Aug;34(8):766-70.1. Flavonoids are naturally occurring compounds that possess anti-allergic, anti-inflammatory, antiproliferative and anti-oxidant properties.
|
| Structure Identification | J AOAC Int. 2009 May-Jun;92(3):789-96.Liquid chromatographic determination of narirutin and hesperidin in Zhi Ke (Citrus aurantium L.) in the form of the raw herb and of the dried aqueous extract.[Pubmed: 19610369]A validated analytical method is reported for the analysis of Narirutin and hesperidin in Zhi Ke (Citrus aurantium L.) in the form of the dried raw herb and of the commercially prepared dried aqueous extract.
|

Narirutin Dilution Calculator

Narirutin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7226 mL | 8.6128 mL | 17.2256 mL | 34.4513 mL | 43.0641 mL |
| 5 mM | 0.3445 mL | 1.7226 mL | 3.4451 mL | 6.8903 mL | 8.6128 mL |
| 10 mM | 0.1723 mL | 0.8613 mL | 1.7226 mL | 3.4451 mL | 4.3064 mL |
| 50 mM | 0.0345 mL | 0.1723 mL | 0.3445 mL | 0.689 mL | 0.8613 mL |
| 100 mM | 0.0172 mL | 0.0861 mL | 0.1723 mL | 0.3445 mL | 0.4306 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Asperuloside
Catalog No.:BCN6231
CAS No.:14259-45-1
- Calanolide E
Catalog No.:BCN6230
CAS No.:142566-61-8
- Glyasperin D
Catalog No.:BCN6836
CAS No.:142561-10-2
- A 484954
Catalog No.:BCC6203
CAS No.:142557-61-7
- Sageone
Catalog No.:BCN3144
CAS No.:142546-15-4
- Cimidahurinine
Catalog No.:BCN6229
CAS No.:142542-89-0
- 1,2,3,4,7-Pentamethoxy-9H-xanthen-9-one
Catalog No.:BCN1570
CAS No.:14254-96-7
- L-690,330
Catalog No.:BCC5666
CAS No.:142523-38-4
- L-690,488
Catalog No.:BCC5667
CAS No.:142523-14-6
- MK-5172 sodium salt
Catalog No.:BCC1765
CAS No.:1425038-27-2
- 19-Nortestosterone acetate
Catalog No.:BCC8445
CAS No.:1425-10-1
- Glyasperin A
Catalog No.:BCN6228
CAS No.:142474-52-0
- Didymin
Catalog No.:BCN3327
CAS No.:14259-47-3
- Deacetylasperulosidic acid
Catalog No.:BCN3323
CAS No.:14259-55-3
- Daphylloside
Catalog No.:BCN6232
CAS No.:14260-99-2
- Macrocarpal C
Catalog No.:BCN6233
CAS No.:142628-53-3
- Macrocarpal E
Catalog No.:BCN6234
CAS No.:142628-54-4
- Macrocarpal D
Catalog No.:BCN6235
CAS No.:142647-71-0
- 6-Hydroxykaempferol 3,6-diglucoside
Catalog No.:BCN3335
CAS No.:142674-16-6
- Genkwanol B
Catalog No.:BCN8013
CAS No.:142674-67-7
- Macrocarpal B
Catalog No.:BCN6236
CAS No.:142698-60-0
- CP 100356 hydrochloride
Catalog No.:BCC7882
CAS No.:142715-48-8
- Dihydrocurcumenone
Catalog No.:BCN3557
CAS No.:142717-57-5
- FGIN-1-27
Catalog No.:BCC6738
CAS No.:142720-24-9
Narirutin inhibits airway inflammation in an allergic mouse model.[Pubmed:17600554]
Clin Exp Pharmacol Physiol. 2007 Aug;34(8):766-70.
1. Flavonoids are naturally occurring compounds that possess anti-allergic, anti-inflammatory, antiproliferative and anti-oxidant properties. In the present study, we investigated whether the flavonoid Narirutin could reduce airway inflammation in ovalbumin (OVA)-sensitized/challenged NC/Nga mice, a model of allergic eosinophilic airway inflammation. 2. Mice were initially immunized intraperitoneally with OVA on Days 0 and 7 and then challenged with inhaled OVA on Days 14, 15 and 16. In addition, some mice received Narirutin orally at doses of 0.1, 1 or 10 mg/kg bodyweight daily on Days 7-16. 3. At 10 mg/kg, but not 0.1 or 1 mg/kg, Narirutin significantly diminished OVA-induced airway inflammation caused by infiltration of lung tissue with inflammatory and mucus-producing cells, as well as reduced eosinophil counts in the peripheral blood and bronchoalveolar lavage fluid (BALF), interleukin (IL)-4 levels in BALF and IgE levels in serum. 4. The mechanism of the anti-inflammatory effect of Narirutin are likely to be associated with a reduction in the OVA-induced increases of IL-4 and IgE in a murine model of allergic eosinophilic airway inflammation. These findings suggest that Narirutin may be an effective new tool in the treatment of bronchial asthma.
Narirutin fraction from citrus peels attenuates LPS-stimulated inflammatory response through inhibition of NF-kappaB and MAPKs activation.[Pubmed:22813871]
Food Chem Toxicol. 2012 Oct;50(10):3498-504.
In this study, we examined the regulatory activity of Narirutin fraction from citrus peels on the production of inflammatory mediators managing acute or chronic inflammatory diseases in macrophages. Narirutin fraction inhibited the release, by lipopolysaccharide (LPS)-stimulated macrophages, of nitric oxide (NO) and prostaglandin E2 (PGE2) through suppressing the expression of inducible NO synthase (iNOS) and cyclooxygenase-2 (COX-2), respectively. The release, by LPS stimulated macrophages, of interleukin-1beta (IL-1beta) and tumor necrosis factor-alpha (TNF-alpha) was also reduced by Narirutin fraction in a dose-dependent manner. Furthermore, Narirutin fraction inhibited the LPS-mediated activation of nuclear factor-kappaB (NF-kappaB) and mitogen-activated protein kinases (MAPKs), which are signaling molecules involved in production of pro-inflammatory factors. As a result of these properties, Narirutin fraction has the potential to be used as a functional dietary supplement and effective anti-inflammatory agent.
Narirutin fraction from citrus peels attenuates alcoholic liver disease in mice.[Pubmed:23416143]
Food Chem Toxicol. 2013 May;55:637-44.
This study aimed to demonstrate protective activities of the Narirutin fraction from peels of Citrus unshiu against ethanol-induced hepatic damage through an animal study. Citrus Narirutin fraction (CNF), contained 75% of Narirutin, was obtained by an ultra-sonicated extraction and further purification. ICR mice were divided into four groups; normaldiet control, ethanol control (6.5g ethanol/kg), low-CNF (ethanol+150mg CNF/kg) and high-CNF (ethanol+300mg CNF/kg) groups. Consumption of alcohol for 8weeks induced severe liver damage with increases in prognostic indicators such as aspartate transaminase, alanine transaminase in serum whereas co-administration of CNF suppressed their increases. Excessive accumulations in liver TG and TC in ethanol control group were also suppressed by co-administration of CNF. Co-administration of CNF maintained SOD activity, GSH and malondialdehyde levels close to those of the normal diet group. Chronic consumption of alcohol also stimulated abrupt increases in pro-inflammatory cytokines such as nuclear factor (NF)-kappaB, tumor necrosis factor (TNF)-alpha and interleukin (IL)-1beta in liver otherwise co-administration of CNF effectively suppressed production of these cytokines dose-dependently. These results indicate that co-administration of CNF with alcohol can alleviate alcohol induced liver damage through preventing lipid formation, protecting antioxidant system and suppressing productions of pro-inflammatory cytokines.
Liquid chromatographic determination of narirutin and hesperidin in Zhi Ke (Citrus aurantium L.) in the form of the raw herb and of the dried aqueous extract.[Pubmed:19610369]
J AOAC Int. 2009 May-Jun;92(3):789-96.
A validated analytical method is reported for the analysis of Narirutin and hesperidin in Zhi Ke (Citrus aurantium L.) in the form of the dried raw herb and of the commercially prepared dried aqueous extract. The samples were extracted by sonication in methanol and the extract was analyzed by liquid chromatography-photodiode array (PDA) detection with identity confirmation by negative electrospray ionization-tandem mass spectrometry (MS). A C18 column was used with a methanol-water gradient mobile phase. Narirutin and hesperidin were quantified at 284 nm using the PDA detector. Using the MS detector, the Narirutin precursor ion with m/z 579 produced daughter ions with m/z 271 and 151. For hesperidin, the precursor ion with m/z 609 produced the m/z 301, 285, and 164 ions. The amounts of Narirutin and hesperidin found in the certified raw herb were 14.2 and 147.9 mg/g, respectively, and in the dried aqueous extract the amounts were 9.2 and 8.6 mg/g, respectively. For the raw herb, the average recovery across the three spike levels (50, 100, and 150%) for Narirutin and hesperidin were 110.7 and 94.5%, respectively. For the dried aqueous extract, the average recovery across the three spike levels for Narirutin and hesperidin were 85.8 and 98.9%, respectively.


