N-MethylcytisineCAS# 486-86-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 486-86-2 | SDF | Download SDF |
| PubChem ID | 442947 | Appearance | White-pale yellow powder |
| Formula | C12H16N2O | M.Wt | 204.27 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Caulophylline | ||
| Solubility | Soluble in chloroform and methanol; sparingly soluble in acetone | ||
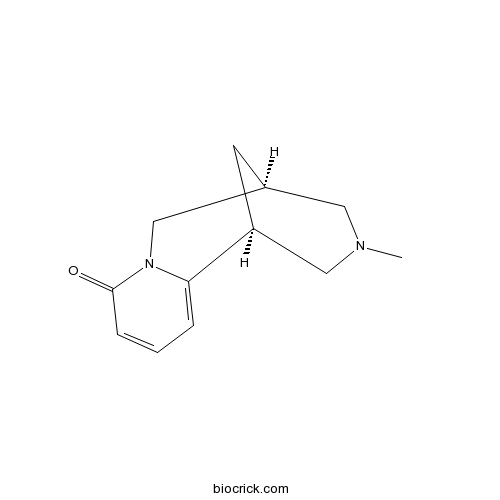
| SMILES | CN1CC2CC(C1)C3=CC=CC(=O)N3C2 | ||
| Standard InChIKey | CULUKMPMGVXCEI-UWVGGRQHSA-N | ||
| Standard InChI | InChI=1S/C12H16N2O/c1-13-6-9-5-10(8-13)11-3-2-4-12(15)14(11)7-9/h2-4,9-10H,5-8H2,1H3/t9-,10-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | N-Methylcytisine's nicotinic receptors have high affinity (KD = 50 nM)to nAChR from squid optical ganglia, N-methylcytisine is a selective ligand of nicotinic receptors of acetylcholine in the central nervous system. (−)-N-methylcytisine and (−)-anagyrine have nematicidal activity against pine wood nematodes. |
| Targets | AChR |
| In vitro | [Effect of nucleotides on N-methylcytisine and dimethyltubocurarine binding by the nicotinic acetylcholine receptors of the optic ganglia in the squid B. magister].[Pubmed: 2790167]Biull Eksp Biol Med. 1989 Jun;107(6):706-9.The effect of nucleosides mono-, di-, and triphosphates on binding of 3H-N-Methylcytisine and 14C-tubocurarine to nAChR from squid optical ganglia were investigated. [N-methylcytisine--a selective ligand of nicotinic receptors of acetylcholine in the CNS].[Pubmed: 3689962]Biull Eksp Biol Med. 1987 Dec;104(12):690-2.The ability of cytisine and its N-methyl derivatives to bind to nicotinic acetylcholine receptors (nAChR) from different tissues was studied. Nematicidal Activities of ( – )--Methylcytisine and ( – )-Anagyrine from Sophora flavescens against Pine Wood Nematodes[Reference: WebLink]Agric. Biol. Chem., 1989, 53(8):2287-8.Although the effects of ( - )-N-methyleytisine (1) on the motility of Angiostrongylus cantonensis, Dipylidium caninum and Fasciola hepatica have already been investigated by Te r ada e t al.,5) the activities of ( -)-N-Methylcytisine (1) and ( -)-anagyrine (2) against pl ant parasitic nematodes have never been reported. |
| Structure Identification | J Chromatogr B Analyt Technol Biomed Life Sci. 2015 May 15;990:118-24.Determination of N-methylcytisine in rat plasma by UPLC-MS/MS and its application to pharmacokinetic study.[Pubmed: 25864013]In this work, a sensitive and selective UPLC-MS/MS method for determination of N-Methylcytisine in rat plasma is developed. Anal Bioanal Chem. 2013 May;405(13):4409-17.Primary constituents of blue cohosh: quantification in dietary supplements and potential for toxicity.[Pubmed: 23420136]Dietary supplements containing dried roots or extracts of the roots and/or rhizomes of blue cohosh (Caulophyllum thalictroides) are widely available. This botanical has a long history of use by Native Americans and its use continues to the present day. |

N-Methylcytisine Dilution Calculator

N-Methylcytisine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.8955 mL | 24.4774 mL | 48.9548 mL | 97.9096 mL | 122.387 mL |
| 5 mM | 0.9791 mL | 4.8955 mL | 9.791 mL | 19.5819 mL | 24.4774 mL |
| 10 mM | 0.4895 mL | 2.4477 mL | 4.8955 mL | 9.791 mL | 12.2387 mL |
| 50 mM | 0.0979 mL | 0.4895 mL | 0.9791 mL | 1.9582 mL | 2.4477 mL |
| 100 mM | 0.049 mL | 0.2448 mL | 0.4895 mL | 0.9791 mL | 1.2239 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Harman
Catalog No.:BCN3998
CAS No.:486-84-0
- Daidzein
Catalog No.:BCN5590
CAS No.:486-66-8
- Vasicinone
Catalog No.:BCN5589
CAS No.:486-64-6
- Isoformononetin
Catalog No.:BCN8206
CAS No.:486-63-5
- Ononin
Catalog No.:BCN5926
CAS No.:486-62-4
- Bergaptol
Catalog No.:BCN5588
CAS No.:486-60-2
- (-)-Cotinine
Catalog No.:BCC7569
CAS No.:486-56-6
- (S)-Coclaurine
Catalog No.:BCN5585
CAS No.:486-39-5
- Daphnetin
Catalog No.:BCN1051
CAS No.:486-35-1
- Fraxinol
Catalog No.:BCN5584
CAS No.:486-28-2
- Isofraxidin
Catalog No.:BCN2327
CAS No.:486-21-5
- PMX 464
Catalog No.:BCC6348
CAS No.:485842-97-5
- alpha-Isolupanine
Catalog No.:BCN7989
CAS No.:486-87-3
- Anagyrine
Catalog No.:BCN3049
CAS No.:486-89-5
- Thermopsine
Catalog No.:BCN2603
CAS No.:486-90-8
- AZD2858
Catalog No.:BCC4509
CAS No.:486424-20-8
- CIQ
Catalog No.:BCC7862
CAS No.:486427-17-2
- O-Acetylcyclocalopin A
Catalog No.:BCN5586
CAS No.:486430-93-7
- Cyclocalopin A
Catalog No.:BCN5587
CAS No.:486430-94-8
- Citropten
Catalog No.:BCN4831
CAS No.:487-06-9
- Elemicin
Catalog No.:BCN2818
CAS No.:487-11-6
- Scopoline
Catalog No.:BCN1942
CAS No.:487-27-4
- Syringaresinol
Catalog No.:BCN3042
CAS No.:487-35-4
- Pinoresinol
Catalog No.:BCN5591
CAS No.:487-36-5
Determination of N-methylcytisine in rat plasma by UPLC-MS/MS and its application to pharmacokinetic study.[Pubmed:25864013]
J Chromatogr B Analyt Technol Biomed Life Sci. 2015 May 15;990:118-24.
In this work, a sensitive and selective UPLC-MS/MS method for determination of N-Methylcytisine in rat plasma is developed. After addition of hordenine as an internal standard (IS), protein precipitation by acetonitrile-methanol (9:1, v/v) was used to prepare samples. Chromatographic separation was achieved on a UPLC BEH HILIC (2.1 mmx100mm, 1.7mum) with acetonitrile (containing 10mM ammonium formate) and water (containing 0.1% formic acid and 10mM ammonium formate) as the mobile phase with gradient elution. An electrospray ionization source was applied and operated in positive ion mode; multiple reaction monitoring (MRM) mode was used for quantification using target fragment ions m/z 205.1-->58.0 for N-Methylcytisine, and m/z 166.1-->121.0 for IS. Calibration plots were linear throughout the range 2-2000ng/mL for N-Methylcytisine in rat plasma. Mean recoveries of N-Methylcytisine in rat plasma ranged from 86.1% to 94.8%. RSD of intra-day and inter-day precision were both<13%. The accuracy of the method was between 94.5% and 109.4%. The method was successfully applied to pharmacokinetic study of N-Methylcytisine after either oral or intravenous administration. For the first time, the absolute bioavailability of N-Methylcytisine was reported as high as 55.5%.
[N-methylcytisine--a selective ligand of nicotinic receptors of acetylcholine in the CNS].[Pubmed:3689962]
Biull Eksp Biol Med. 1987 Dec;104(12):690-2.
The ability of cytisine and its N-methyl derivatives to bind to nicotinic acetylcholine receptors (nAChR) from different tissues was studied. Cytisine and N-Methylcytisine have high affinity (KD = 50 nM) to nAChR from squid optical ganglia. N,N-dimethylcytisine did not show high affinity to this receptor. In the case of nAChR from T. marmorata, cytisine was the only effective inhibitor of 14C-tubocurarine specific binding (Ki = 700 nM). N-methyl- and N,N-dimethylcytisine did not displace 14C-tubocurarine at a concentration of 0.1 mM. The results obtained indicate that there are some differences in the structure of nAChR binding sites from squid and T. marmorata optical ganglia.
Primary constituents of blue cohosh: quantification in dietary supplements and potential for toxicity.[Pubmed:23420136]
Anal Bioanal Chem. 2013 May;405(13):4409-17.
Dietary supplements containing dried roots or extracts of the roots and/or rhizomes of blue cohosh (Caulophyllum thalictroides) are widely available. This botanical has a long history of use by Native Americans and its use continues to the present day. The primary constituents of blue cohosh are its alkaloids and saponins. The structures of the alkaloids magnoflorine, baptifoline, anagyrine, and N-Methylcytisine have been known for many years. The last 10 years have seen a great increase in isolation and identification of the large number of saponins present in blue cohosh. Important developments in nuclear magnetic resonance techniques have contributed substantially to the increase in elucidation of the structures of the complex saponins. Several authors have described quantitative methods for both the alkaloids and saponins in blue cohosh. Such methods have made it possible to quantify these constituents in dietary supplements containing this botanical ingredient. Concentrations of both alkaloids and saponins vary substantially in dietary supplements of blue cohosh. The nicotinic alkaloid, N-Methylcytisine, a potent toxicant, has been found in all dietary supplements of blue cohosh analyzed. The teratogenic alkaloid anagyrine has been found in some but not all dietary supplements.
[Effect of nucleotides on N-methylcytisine and dimethyltubocurarine binding by the nicotinic acetylcholine receptors of the optic ganglia in the squid B. magister].[Pubmed:2790167]
Biull Eksp Biol Med. 1989 Jun;107(6):706-9.
The effect of nucleosides mono-, di-, and triphosphates on binding of 3H-N-Methylcytisine and 14C-tubocurarine to nAChR from squid optical ganglia were investigated. It was found, that ATP and GTP potentiate the specific binding of 3H-N-Methylcytisine and inhibit the one of 14C-tubocurarine. While conducting the photoaffinity modification of nACHR by 3H-azidomethylcytisine in the presence of ATP the increase of specific incorporation of label was observed in comparison with control. Molecular weight of labeled receptor complex and subunit, carrying the binding site was the same as the original.


