IsoformononetinCAS# 486-63-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 486-63-5 | SDF | Download SDF |
| PubChem ID | 3764 | Appearance | Off-white powder |
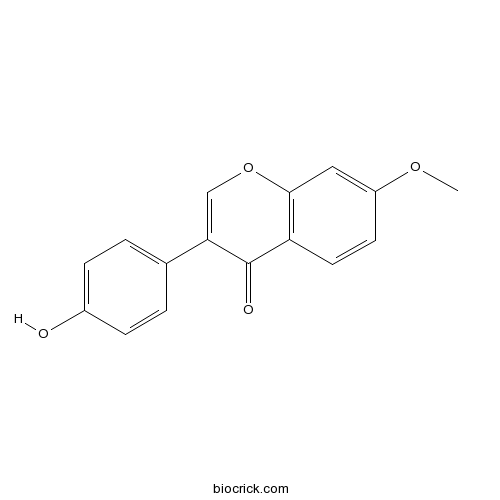
| Formula | C16H12O4 | M.Wt | 268.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | 4'-Hydroxy 7-methoxyisoflavone | ||
| Solubility | Soluble in acetone and methan | ||
| Chemical Name | 3-(4-hydroxyphenyl)-7-methoxychromen-4-one | ||
| SMILES | COC1=CC2=C(C=C1)C(=O)C(=CO2)C3=CC=C(C=C3)O | ||
| Standard InChIKey | LNIQZRIHAMVRJA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H12O4/c1-19-12-6-7-13-15(8-12)20-9-14(16(13)18)10-2-4-11(17)5-3-10/h2-9,17H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Isoformononetin has immunomodulatory activity, it inhibits the differentiation of Th17 cells and B-cell lymphopoesis to promote osteogenesis in estrogen-deficient bone loss conditions. 2. Isoformononetin reverses bone loss in osteopenic rats and exerts bone anabolic action by preventing osteoblast apoptosis. 3. Isoformononetin shows fungitoxic activity against Cladosporium sphaerospermum. |
| Targets | IL Receptor | Antifection |

Isoformononetin Dilution Calculator

Isoformononetin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7272 mL | 18.6359 mL | 37.2717 mL | 74.5434 mL | 93.1793 mL |
| 5 mM | 0.7454 mL | 3.7272 mL | 7.4543 mL | 14.9087 mL | 18.6359 mL |
| 10 mM | 0.3727 mL | 1.8636 mL | 3.7272 mL | 7.4543 mL | 9.3179 mL |
| 50 mM | 0.0745 mL | 0.3727 mL | 0.7454 mL | 1.4909 mL | 1.8636 mL |
| 100 mM | 0.0373 mL | 0.1864 mL | 0.3727 mL | 0.7454 mL | 0.9318 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ononin
Catalog No.:BCN5926
CAS No.:486-62-4
- Bergaptol
Catalog No.:BCN5588
CAS No.:486-60-2
- (-)-Cotinine
Catalog No.:BCC7569
CAS No.:486-56-6
- (S)-Coclaurine
Catalog No.:BCN5585
CAS No.:486-39-5
- Daphnetin
Catalog No.:BCN1051
CAS No.:486-35-1
- Fraxinol
Catalog No.:BCN5584
CAS No.:486-28-2
- Isofraxidin
Catalog No.:BCN2327
CAS No.:486-21-5
- PMX 464
Catalog No.:BCC6348
CAS No.:485842-97-5
- Mirabijalone D
Catalog No.:BCN4071
CAS No.:485811-84-5
- Choline sulphate
Catalog No.:BCN1792
CAS No.:4858-96-2
- Proanthocyanidins
Catalog No.:BCN6313
CAS No.:4852-22-6
- 5,7,3'-Trihydroxyflavanone
Catalog No.:BCC8269
CAS No.:104732-07-2
- Vasicinone
Catalog No.:BCN5589
CAS No.:486-64-6
- Daidzein
Catalog No.:BCN5590
CAS No.:486-66-8
- Harman
Catalog No.:BCN3998
CAS No.:486-84-0
- N-Methylcytisine
Catalog No.:BCN1266
CAS No.:486-86-2
- alpha-Isolupanine
Catalog No.:BCN7989
CAS No.:486-87-3
- Anagyrine
Catalog No.:BCN3049
CAS No.:486-89-5
- Thermopsine
Catalog No.:BCN2603
CAS No.:486-90-8
- AZD2858
Catalog No.:BCC4509
CAS No.:486424-20-8
- CIQ
Catalog No.:BCC7862
CAS No.:486427-17-2
- O-Acetylcyclocalopin A
Catalog No.:BCN5586
CAS No.:486430-93-7
- Cyclocalopin A
Catalog No.:BCN5587
CAS No.:486430-94-8
- Citropten
Catalog No.:BCN4831
CAS No.:487-06-9
Effect of spores of saprophytic fungi on phytoalexin accumulation in seeds of frog-eye leaf spot and stem canker-resistant and -susceptible soybean (Glycine max L.) cultivars.[Pubmed:10956166]
J Agric Food Chem. 2000 Aug;48(8):3662-5.
Two saprophytic fungi (Mucor ramosissimus and Rhizopus sp.) were tested for their ability to induce phytoalexin production by seeds of frog-eye leaf spot and stem canker-resistant and -susceptible soybean (Glycine max L.) cultivars. Only M. ramosissimus was shown to elicit a response and qualitative differences in phytoalexin accumulation were found between the susceptible and resistant cultivars. Glyceollins I, II, and III and glycinol were isolated from the susceptible cultivar, whereas Glyceollins I, II, and III, glycinol, glyceocarpin, genistein, Isoformononetin, and N-acetyltyramine accumulated in the resistant cultivar in response to the same fungal elicitor. Genistein was found to be an inducibly formed isoflavonoid instead of a constitutive metabolite in the resistant cultivar, whereas N-acetyltyramine is described for the first time as a soybean phytoalexin. All the compounds, except genistein, showed fungitoxic activity against Cladosporium sphaerospermum. Spectral data of the pterocarpan phytoalexins, genistein, and N-acetyltyramine are also given in this work.
Methoxyisoflavones formononetin and isoformononetin inhibit the differentiation of Th17 cells and B-cell lymphopoesis to promote osteogenesis in estrogen-deficient bone loss conditions.[Pubmed:27070807]
Menopause. 2016 May;23(5):565-76.
OBJECTIVE: Recent studies have shown that immune system plays a major role in pathophysiology of postmenopausal osteoporosis. Previously we have shown that phytoestrogens like daidzein and medicarpin exhibit immunoprotective effects, by virtue of which they alleviate bone loss. With this background, methoxyisoflavones like formononetin (formo) and Isoformononetin (isoformo) that have been studied for preventing bone loss in ovariectomized rats were tested for their immunomodulatory effects in estrogen-deficient bone loss mice model. METHODS: Adult Balb/c mice (N = 8/group) were given oral dose of formo and isoformo at 10 mg/kg body weight, post ovariectomy (Ovx) daily for 6 weeks. Animals were autopsied and long bones were harvested to study bone microarchitecture. Peripheral blood mononuclear cells were isolated for fluorescence-activated cell sorting and RNA analysis. Serum was collected for enzyme-linked immunosorbent assay. RESULTS: It was observed that formo and isoformo treatment to Ovx mice led to significant restoration of Ovx-induced deterioration of trabecular microarchitecture. Pro-osteoclastogenic subset Th17 and B cells were decreased in formo/isoformo-treated Ovx mice in comparison with vehicle-treated Ovx group. Formo and isoformo treatment to Ovx mice also led to decreased expression of Th17 diffentiation factors and promoted T-regulatory cell differentiation. Formo was more effective in enhancing the FOXP3 expression compared with isoformo. IL-17A-induced osteoclastogenesis and inhibition of osteoblast apoptosis were also suppressed by formo and isoformo treatment, with formo having a more potent effect. CONCLUSIONS: Our study demonstrates the immunomodulatory activity of methoxyisoflavones, formo, and isoformo, which translate into improved skeletal parameters, thereby preventing Ovx-induced bone loss.
Chemical constituents of Arisaema franchetianum tubers.[Pubmed:23106482]
J Asian Nat Prod Res. 2013;15(1):71-7.
A novel pyrrolidine alkaloid, (2R*,3S*,5S*)-N,2-dimethyl-3-hydroxy-5-(10-phenyldecyl)pyrrolidine (1), and 17 known compounds were isolated from Arisaema franchetianum Engl. (Araceae) tubers. The 17 compounds were bergenin (2), emodin (3), caffeic acid (4), nobiletin (5), 3-O-beta-d-galactopyranosyl-hederagenin 28-O-beta-d-xylopyranosyl(1 --> 6)-beta-d-galactopyranosyl ester (6), coniferin (7), qingyangshengenin (8), methylconiferin (9), syringaresinol 4'-O-beta-d-glucopyranoside (10), gagaminine (11), perlolyrine (12), (S)-1-(1'-hydroxyethyl)-beta-carboline (13), 1-(beta-carboline-1-yl)-3,4,5-trihydroxy-1-pentanone (14), 1-methoxycarbonyl-beta-carboline (15), indolo[2,3-alpha]carbazole (16), 4-hydroxycinnamic acid methyl ester (17), and methyl 4-[2-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-1-(hydroxymethyl)ethyl] ferulate (18). The inhibitory activities of compound 1 and its N-methyl derivative (1a) against porcine respiratory and reproductive syndrome virus (PRRSV), human leukemic K562 cells, and human breast cancer MCF-7 cells were evaluated. Compounds 1 [50% inhibited concentration (IC(50)) = 12.5 +/- 0.6 muM] and 1a (IC(50) = 15.7 +/- 0.9 muM) were cytotoxic against K562 cells. Compound 1a also had a weak effect on PRRSV with an IC(50) value of 31.9 +/- 6.0 muM [selectivity index (SI) = 18.7].
Isoformononetin, a methoxydaidzein present in medicinal plants, reverses bone loss in osteopenic rats and exerts bone anabolic action by preventing osteoblast apoptosis.[Pubmed:23395215]
Phytomedicine. 2013 Apr 15;20(6):470-80.
PURPOSE: Daidzein (Daid) has been implicated in bone health for its estrogen-'like' effects but low bioavailability, unfavorable metabolism and uterine estrogenicity impede its clinical potential. This study was aimed at assessing Isoformononetin (Isoformo), a naturally occurring methoxydaidzein, for bone anabolic effect by overcoming the pitfalls associated with Daid. METHODS: Sprague-Dawley ovariectomized (OVx) rats with established osteopenia were administered Isoformo, 17beta-oestradiol (E2) or human parathyroid hormone. Efficacy was evaluated by bone microarchitecture using microcomputed tomography and determination of new bone formation by fluorescent labeling of bone. Osteoblast apoptosis was measured by co-labeling of bone sections with Runx-2 and TUNEL. Biochemical markers of bone metabolism were measured by ELISA. Plasma and bone marrow levels of Isoformo and Daid were determined by LC-MS-MS. Rat bone marrow stromal cells were harvested to study osteoblastic differentiation by Isoformo and Daid. New born rat pups were injected with Isoformo and Daid to study the effect of the compounds on the expression of osteogenic genes in the calvaria by real time PCR. RESULTS: In osteopenic rats, Isoformo treatment restored trabecular microarchitecture, increased new bone formation, increased the serum osteogenic marker (procollagen N-terminal propeptide), decreased resorptive marker (urinary C-terminal teleopeptide of type I collagen) and diminished osteoblast apoptosis in bone. At the most effective osteogenic dose of Isoformo, plasma and bone marrow levels were comprised of ~90% Isoformo and the rest, Daid. Isoformo at the concentration reaching the bone marrow achieved out of its most effective oral dosing induced stromal cell mineralization and osteogenic gene expression in the calvaria of neonatal rats. Isoformo exhibited uterine safety. CONCLUSIONS: Our study demonstrates that Isoformo reverses established osteopenia in adult OVx rats likely via its pro-survival effect on osteoblasts. Given its bone anabolic and anti-catabolic effects accompanied with safety at uterine level we propose its potential in the management of postmenopausal osteoporosis.


