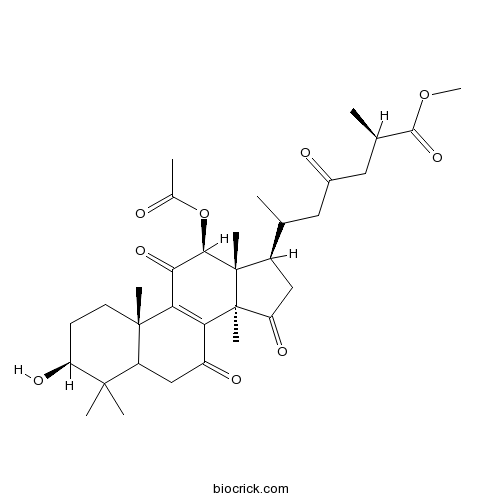
Methyl ganoderate HCAS# 98665-11-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 98665-11-3 | SDF | Download SDF |
| PubChem ID | 102004759 | Appearance | Powder |
| Formula | C33H46O9 | M.Wt | 586.7 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | methyl (2R)-6-[(3S,10S,12S,13R,14R,17R)-12-acetyloxy-3-hydroxy-4,4,10,13,14-pentamethyl-7,11,15-trioxo-1,2,3,5,6,12,16,17-octahydrocyclopenta[a]phenanthren-17-yl]-2-methyl-4-oxoheptanoate | ||
| SMILES | CC(CC(=O)CC(C)C(=O)OC)C1CC(=O)C2(C1(C(C(=O)C3=C2C(=O)CC4C3(CCC(C4(C)C)O)C)OC(=O)C)C)C | ||
| Standard InChIKey | KXYWXCIDKNDYTK-NIOCUZMRSA-N | ||
| Standard InChI | InChI=1S/C33H46O9/c1-16(12-19(35)13-17(2)29(40)41-9)20-14-24(38)33(8)25-21(36)15-22-30(4,5)23(37)10-11-31(22,6)26(25)27(39)28(32(20,33)7)42-18(3)34/h16-17,20,22-23,28,37H,10-15H2,1-9H3/t16?,17-,20-,22?,23+,28-,31+,32+,33+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Methyl ganoderate H Dilution Calculator

Methyl ganoderate H Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7044 mL | 8.5222 mL | 17.0445 mL | 34.089 mL | 42.6112 mL |
| 5 mM | 0.3409 mL | 1.7044 mL | 3.4089 mL | 6.8178 mL | 8.5222 mL |
| 10 mM | 0.1704 mL | 0.8522 mL | 1.7044 mL | 3.4089 mL | 4.2611 mL |
| 50 mM | 0.0341 mL | 0.1704 mL | 0.3409 mL | 0.6818 mL | 0.8522 mL |
| 100 mM | 0.017 mL | 0.0852 mL | 0.1704 mL | 0.3409 mL | 0.4261 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Zedoarondiol
Catalog No.:BCN3560
CAS No.:98644-24-7
- Nitrocaramiphen hydrochloride
Catalog No.:BCC6655
CAS No.:98636-73-8
- Sarmentocymarin
Catalog No.:BCN7489
CAS No.:98633-61-5
- (S)-(-)-Bay K 8644
Catalog No.:BCC7108
CAS No.:98625-26-4
- Schisandrone
Catalog No.:BCN3316
CAS No.:98619-25-1
- Cryptanoside A
Catalog No.:BCN7481
CAS No.:98570-81-1
- Sominone
Catalog No.:BCN8006
CAS No.:98569-64-3
- 4-Chloro-6-iodoquinazoline
Catalog No.:BCC8703
CAS No.:98556-31-1
- Methyl 2-bromomethyl-3-nitrobenzoate
Catalog No.:BCC9035
CAS No.:98475-07-1
- Pseudoginsenoside RT5
Catalog No.:BCN1076
CAS No.:98474-78-3
- Pseudoginsenoside RT1
Catalog No.:BCN2794
CAS No.:98474-74-9
- Myelin Basic Protein (68-82), guinea pig
Catalog No.:BCC1020
CAS No.:98474-59-0
- Ganoderic acid F
Catalog No.:BCN3037
CAS No.:98665-14-6
- Ganoderic Acid J
Catalog No.:BCN8436
CAS No.:100440-26-4
- Lucidenic acid D2
Catalog No.:BCN8202
CAS No.:98665-16-8
- Ganoderic acid H
Catalog No.:BCN3038
CAS No.:98665-19-1
- Ganoderic acid I
Catalog No.:BCN2865
CAS No.:98665-20-4
- Ganolucidic acid A
Catalog No.:BCN2444
CAS No.:98665-21-5
- Ganoderic acid G
Catalog No.:BCN2915
CAS No.:98665-22-6
- Dregeoside Da1
Catalog No.:BCN4764
CAS No.:98665-65-7
- Dregeoside Ga1
Catalog No.:BCN4548
CAS No.:98665-66-8
- ATP disodium salt
Catalog No.:BCC5160
CAS No.:987-65-5
- Ropivacaine HCl
Catalog No.:BCC4841
CAS No.:98717-15-8
- Paeonilactone C
Catalog No.:BCN3964
CAS No.:98751-77-0
A novel assessment of the role of the methyl radical and water formation channel in the CH3OH + H reaction.[Pubmed:28890979]
Phys Chem Chem Phys. 2017 Sep 20;19(36):24467-24477.
A number of experimental and theoretical papers accounted almost exclusively for two channels in the reaction of atomic hydrogen with methanol: H-abstraction from the methyl (R1) and hydroxyl (R2) functional groups. Recently, several astrochemical studies claimed the importance of another channel for this reaction, which is crucial for kinetic simulations related to the abundance of molecular constituents in planetary atmospheres: methyl radical and water formation (R3 channel). Here, motivated by the lack of and uncertainties about the experimental and theoretical kinetic rate constants for the third channel, we developed first-principles Car-Parrinello molecular dynamics thermalized at two significant temperatures - 300 and 2500 K. Furthermore, the kinetic rate constant of all three channels was calculated using a high-level deformed-transition state theory (d-TST) at a benchmark electronic structure level. d-TST is shown to be suitable for describing the overall rate constant for the CH3OH + H reaction (an archetype of the moderate tunnelling regime) with the precision required for practical applications. Considering the experimental ratios at 1000 K, kR1/kR2 approximately 0.84 and kR1/kR3 approximately 15-40, we provided a better estimate when compared with previous theoretical work: 7.47 and 637, respectively. The combination of these procedures explicitly demonstrates the role of the third channel in a significant range of temperatures and indicates its importance considering the thermodynamic control to estimate methyl radical and water formation. We expect that these results can help to shed new light on the fundamental kinetic rate equations for the CH3OH + H reaction.
Crystal structure of {(R)-N(2)-[(benzo[h]quinolin-2-yl)meth-yl]-N(2')-[(benzo[h]quinolin-2-yl)methyl-i dene]-1,1'-binaphthyl-2,2'-di-amine-kappa(4)N,N',N'',N'''}(trifluoromethane-sulfo nato-kappaO)zinc(II)} trifluoromethane-sulfonate di-chloro-methane 1.5-solvate.[Pubmed:28775858]
Acta Crystallogr E Crystallogr Commun. 2017 Jun 2;73(Pt 7):949-953.
The zinc(II) atom in the title compound, [Zn(C48H31N4)(CF3SO3)](CF3SO3).1.5CH2Cl2, adopts a distorted five-coordinate square-pyramidal geometry. It is coordinated by one tri-fluoro-methane-sulfonate ligand and four N atoms of the N(2)-[(benzo[h]quinolin-2-yl)meth-yl]-N(2')-[(benzo[h]quinolin-2-yl)methyl-idene] -1,1'-binaphthyl-2,2'-di-amine ligand. The complex is present as a single-stranded P-helimer monohelical structure incorporating pi-pi and/or sigma-pi inter-actions. One of the imine bonds present in the original ligand framework is reduced, leading to variations in bond lengths and torsion angles for each side of the ligand motif. The imine-bond reduction also affects the bond lengths involving the metal atom with the N-donor atoms located on the imine bond. There are two mol-ecules of the complex in the asymmetric unit. One of the mol-ecules exhibits positional disorder within the coordinating tri-fluoro-methane-sulfonate ion making the mol-ecules symmetric-ally non-equivalent.
Nucleoside-O-Methyl-(H)-Phosphinates: Novel Monomers for the Synthesis of Methylphosphonate Oligonucleotides Using H-Phosphonate Chemistry.[Pubmed:28921496]
Curr Protoc Nucleic Acid Chem. 2017 Sep 18;70:4.76.1-4.76.22.
This unit comprises the straightforward synthesis of protected 2'-deoxyribonucleoside-O-methyl-(H)-phosphinates in both 3'- and 5'-series. These compounds represent a new class of monomers compatible with the solid-phase synthesis of oligonucleotides using H-phosphonate chemistry and are suitable for the preparation of both 3'- and 5'-O-methylphosphonate oligonucleotides. The synthesis of 4-toluenesulfonyloxymethyl-(H)-phosphinic acid as a new reagent for the preparation of O-methyl-(H)-phosphinic acid derivatives is described. (c) 2017 by John Wiley & Sons, Inc.
Development of a ReaxFF Force Field for Cu/S/C/H and Reactive MD Simulations of Methyl Thiolate Decomposition on Cu (100).[Pubmed:28981284]
J Phys Chem B. 2018 Jan 18;122(2):888-896.
It has been shown that the rate of decomposition of methyl thiolate species on copper is accelerated by sliding on a methyl thiolate covered surface in ultrahigh vacuum at room temperature. The reaction produces small gas-phase hydrocarbons and deposits sulfur on the surface. Here, a new ReaxFF potential was developed to enable investigation of the molecular processes that induce this mechanochemical reaction by using density functional theory calculations to tune force field parameters for the model system. Various processes, including volumetric expansion/compression of CuS, CuS2, and Cu2S unit cells; bond dissociation of Cu-S and valence angle bending of Cu-S-C; the binding energies of SCH3, CH3, and S atoms on a Cu surface; and energy for the decomposition of methyl thiolate molecular species on copper, were used to identify the new ReaxFF parameters. Molecular dynamics simulations of the reactions of adsorbed methyl thiolate species at various temperatures were performed to demonstrate the validity of the new potential and to study the thermal reaction pathways. It was found that reaction is initiated by C-S bond scission, consistent with experiments, and that the resulting methyl species diffuse on the surface and combine to desorb ethane, also as found experimentally.
Ir-Catalyzed Enantioselective, Intramolecular Silylation of Methyl C-H Bonds.[Pubmed:28820264]
J Am Chem Soc. 2017 Sep 6;139(35):12137-12140.
We report highly enantioselective intramolecular, silylations of unactivated, primary C(sp(3))-H bonds. The reactions form dihydrobenzosiloles in high yields with excellent enantioselectivities by functionalization of enantiotopic methyl groups under mild conditions. The reaction is catalyzed by an iridium complex generated from [Ir(COD)OMe]2 and chiral dinitrogen ligands that we recently disclosed. The C-Si bonds in the enantioenriched dihydrobenzosiloles were further transformed to C-Cl, C-Br, C-I, and C-O bonds in final products. The potential of this reaction was illustrated by sequential C(sp(3))-H and C(sp(2))-H silylations and functionalizations, as well as diastereoselective C-H silylations of a chiral, natural-product derivative containing multiple types of C-H bonds. Preliminary mechanistic studies suggest that C-H cleavage is the rate-determining step.


