Ganoderic acid ICAS# 98665-20-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 98665-20-4 | SDF | Download SDF |
| PubChem ID | 20055990 | Appearance | Powder |
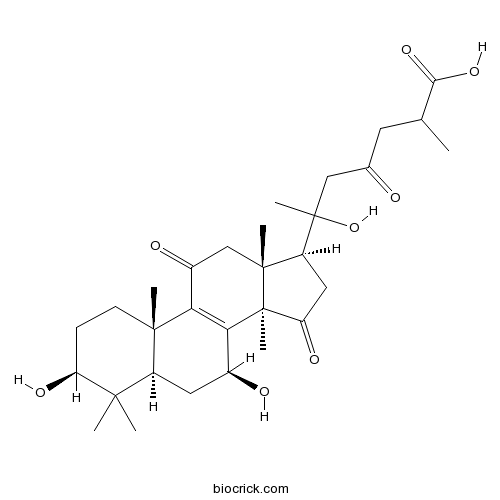
| Formula | C30H44O8 | M.Wt | 532.67 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 6-[(3S,5R,7S,10S,13R,14R,17S)-3,7-dihydroxy-4,4,10,13,14-pentamethyl-11,15-dioxo-2,3,5,6,7,12,16,17-octahydro-1H-cyclopenta[a]phenanthren-17-yl]-6-hydroxy-2-methyl-4-oxoheptanoic acid | ||
| SMILES | CC(CC(=O)CC(C)(C1CC(=O)C2(C1(CC(=O)C3=C2C(CC4C3(CCC(C4(C)C)O)C)O)C)C)O)C(=O)O | ||
| Standard InChIKey | ZWMMEKXOLCCKLA-XDKAHJIMSA-N | ||
| Standard InChI | InChI=1S/C30H44O8/c1-15(25(36)37)10-16(31)13-29(6,38)20-12-22(35)30(7)24-17(32)11-19-26(2,3)21(34)8-9-27(19,4)23(24)18(33)14-28(20,30)5/h15,17,19-21,32,34,38H,8-14H2,1-7H3,(H,36,37)/t15?,17-,19-,20-,21-,27-,28+,29?,30-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Ganoderic acid I is a natural product from Ganoderma lucidum . |
| Structure Identification | Chemical & pharmaceutical bulletin 33(6), 2628-2631, 1985-06-25GANODERIC ACID G AND I AND GANOLUCIDIC ACID A AND B, NEW TRITERPENOIDS FROM GANODERMA LUCIDUM[Reference: WebLink]Four new highly oxidized lanostane-type triterpenoids, ganoderic acid G and Ganoderic acid I and ganolucidic acid A and B, were isolated from the fungus ganoderma lucidum and their structures were elucidated on the basis of spectral evidence. |

Ganoderic acid I Dilution Calculator

Ganoderic acid I Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8773 mL | 9.3867 mL | 18.7733 mL | 37.5467 mL | 46.9334 mL |
| 5 mM | 0.3755 mL | 1.8773 mL | 3.7547 mL | 7.5093 mL | 9.3867 mL |
| 10 mM | 0.1877 mL | 0.9387 mL | 1.8773 mL | 3.7547 mL | 4.6933 mL |
| 50 mM | 0.0375 mL | 0.1877 mL | 0.3755 mL | 0.7509 mL | 0.9387 mL |
| 100 mM | 0.0188 mL | 0.0939 mL | 0.1877 mL | 0.3755 mL | 0.4693 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ganoderic acid H
Catalog No.:BCN3038
CAS No.:98665-19-1
- Lucidenic acid D2
Catalog No.:BCN8202
CAS No.:98665-16-8
- Ganoderic Acid J
Catalog No.:BCN8436
CAS No.:100440-26-4
- Ganoderic acid F
Catalog No.:BCN3037
CAS No.:98665-14-6
- Methyl ganoderate H
Catalog No.:BCN3258
CAS No.:98665-11-3
- Zedoarondiol
Catalog No.:BCN3560
CAS No.:98644-24-7
- Nitrocaramiphen hydrochloride
Catalog No.:BCC6655
CAS No.:98636-73-8
- Sarmentocymarin
Catalog No.:BCN7489
CAS No.:98633-61-5
- (S)-(-)-Bay K 8644
Catalog No.:BCC7108
CAS No.:98625-26-4
- Schisandrone
Catalog No.:BCN3316
CAS No.:98619-25-1
- Cryptanoside A
Catalog No.:BCN7481
CAS No.:98570-81-1
- Sominone
Catalog No.:BCN8006
CAS No.:98569-64-3
- Ganolucidic acid A
Catalog No.:BCN2444
CAS No.:98665-21-5
- Ganoderic acid G
Catalog No.:BCN2915
CAS No.:98665-22-6
- Dregeoside Da1
Catalog No.:BCN4764
CAS No.:98665-65-7
- Dregeoside Ga1
Catalog No.:BCN4548
CAS No.:98665-66-8
- ATP disodium salt
Catalog No.:BCC5160
CAS No.:987-65-5
- Ropivacaine HCl
Catalog No.:BCC4841
CAS No.:98717-15-8
- Paeonilactone C
Catalog No.:BCN3964
CAS No.:98751-77-0
- Paeonilactone B
Catalog No.:BCN3963
CAS No.:98751-78-1
- Paeonilactone A
Catalog No.:BCN3967
CAS No.:98751-79-2
- Latifoline N-oxide
Catalog No.:BCN1979
CAS No.:98752-06-8
- Isothymusin
Catalog No.:BCN4532
CAS No.:98755-25-0
- Reboxetine mesylate
Catalog No.:BCC4934
CAS No.:98769-84-7
Shedding light on the mechanisms underlying the environmental regulation of secondary metabolite ganoderic acid in Ganoderma lucidum using physiological and genetic methods.[Pubmed:30951869]
Fungal Genet Biol. 2019 Apr 2;128:43-48.
The secondary metabolites of fungi are often produced at very low concentrations, and until recently the regulatory mechanisms of secondary metabolite biosynthesis have been unclear. Ganoderma lucidum is a macrofungus that is widely used as a traditional Chinese medicine or medicinal mushroom: ganoderic acid (GA) is one of the main active ingredients. Here, we review research from the last decade on which and how environmental factors regulate GA biosynthesis. These environmental factors are mainly three components: a single chemical/biological or biochemical signal, physical triggers, and nutritional conditions. Because G. lucidum is a non-model Basidiomycete, a combination of physiological and genetic research is needed to determine how those environmental factors regulate GA biosynthesis. The regulation of GA biosynthesis includes ROS, Ca(2+), cAMP and phospholipid signaling, and cross-talk between different signaling pathways. The regulatory mechanisms for the synthesis of this secondary metabolite, from the perspective of physiology and genetics, in G. lucidum will provide ideas for studying the regulation of fungal secondary metabolism in other non-model species, especially those fungi with limitations in genetic manipulation.
Antioxidant potential of ganoderic acid in Notch-1 protein in neuroblastoma.[Pubmed:30511344]
Mol Cell Biochem. 2018 Dec 3. pii: 10.1007/s11010-018-3485-7.
Neuroblastoma is a childhood tumor arising from developing a sympathetic nervous system and causes around 10% of pediatric tumors. Despite advancement in the use of sophisticated techniques in molecular biology, neuroblastoma patient's survivability rate is very less. Notch pathway is significant in upholding cell maintenance and developmental process of organs. Notch-1 proteins are a ligand-activated transmembrane receptor which decides the fate of the cell. Notch signaling leads to transcription of genes which indulged in numerous diseases including tumor progression. Ganoderic acid, a lanosterol triterpene, isolated from fungus Ganoderma lucidum with a wide range of medicinal values. In the present study, various isoforms of the ganoderic acid and natural inhibitors were docked by molecular docking using Maestro 9 in the Notch-1 signaling pathway. The receptor-based molecular docking exposed the best binding interaction of Notch-1 with ganoderic acid A with GScore (- 8.088), kcal/mol, Lipophilic EvdW (- 1.74), Electro (- 1.18), Glide emodel (- 89.944) with the active participation of Arg 189, Arg 199, Glu 232 residues. On the other hand natural inhibitor, curcumin has GScore (- 7.644), kcal/mol, Lipophilic EvdW (- 2.19), Electro (- 0.73), Glide emodel (- 70.957) with Arg 75 residues involved in docking. The ligand binding affinity of ganoderic acid A in Notch-1 is calculated using MM-GBSA (- 76.782), whereas curcumin has (- 72.815) kcal/mol. The QikProp analyzed the various drug-likeness parameters such as absorption, distribution, metabolism, excretion, and toxicity (ADME/T) and isoforms of ganoderic acid require some modification to fall under Lipinski rule. The ganoderic acid A and curcumin were the best-docked among different compounds and exhibits downregulation in Notch-1 mRNA expression and inhibits proliferation, viability, and ROS activity in IMR-32 cells.


