Makisterone ACAS# 20137-14-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 20137-14-8 | SDF | Download SDF |
| PubChem ID | 12312690 | Appearance | Powder |
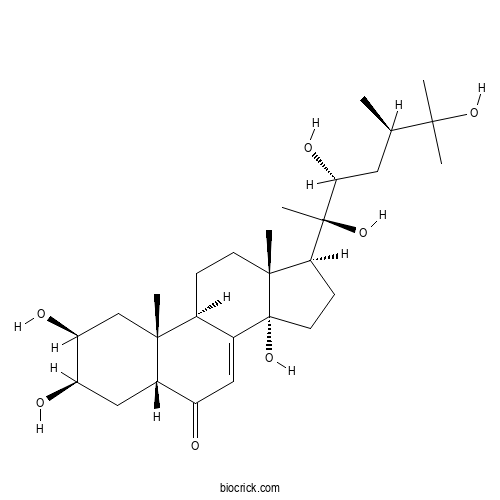
| Formula | C28H46O7 | M.Wt | 494.7 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S,3R,5R,9R,10R,13R,14S,17S)-2,3,14-trihydroxy-10,13-dimethyl-17-[(2R,3R,5R)-2,3,6-trihydroxy-5,6-dimethylheptan-2-yl]-2,3,4,5,9,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-6-one | ||
| SMILES | CC(CC(C(C)(C1CCC2(C1(CCC3C2=CC(=O)C4C3(CC(C(C4)O)O)C)C)O)O)O)C(C)(C)O | ||
| Standard InChIKey | IJRBORPEVKCEQD-JMQWOFAPSA-N | ||
| Standard InChI | InChI=1S/C28H46O7/c1-15(24(2,3)33)11-23(32)27(6,34)22-8-10-28(35)17-12-19(29)18-13-20(30)21(31)14-25(18,4)16(17)7-9-26(22,28)5/h12,15-16,18,20-23,30-35H,7-11,13-14H2,1-6H3/t15-,16+,18+,20-,21+,22+,23-,25-,26-,27-,28-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Makisterone A Dilution Calculator

Makisterone A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0214 mL | 10.1071 mL | 20.2143 mL | 40.4285 mL | 50.5357 mL |
| 5 mM | 0.4043 mL | 2.0214 mL | 4.0429 mL | 8.0857 mL | 10.1071 mL |
| 10 mM | 0.2021 mL | 1.0107 mL | 2.0214 mL | 4.0429 mL | 5.0536 mL |
| 50 mM | 0.0404 mL | 0.2021 mL | 0.4043 mL | 0.8086 mL | 1.0107 mL |
| 100 mM | 0.0202 mL | 0.1011 mL | 0.2021 mL | 0.4043 mL | 0.5054 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Brasilixanthone B
Catalog No.:BCX0228
CAS No.:84002-57-3
- 22-Epiinotodiol
Catalog No.:BCX0227
CAS No.:64907-49-9
- 1,3,5,7-Tetrahydroxy-8-prenylxanthone
Catalog No.:BCX0226
CAS No.:444004-76-6
- 1,6,8-Trihydroxy-2,7-dimethoxy-3-methylanthraquinone
Catalog No.:BCX0225
CAS No.:2366153-27-5
- Phenacetamide
Catalog No.:BCX0224
CAS No.:103-81-1
- 4-(1-Ethoxy-2-hydroxyethyl)benzene-1,2-diol
Catalog No.:BCX0223
CAS No.:1190632-33-7
- 3β,5α-Dihydroxystigmastan-6-one
Catalog No.:BCX0222
CAS No.:55051-78-0
- Fornicin A
Catalog No.:BCX0221
CAS No.:908588-41-0
- Cyclo(L-Pro-L-Ile)
Catalog No.:BCX0220
CAS No.:57089-60-8
- Cyclo(L-Pro-L-Val)
Catalog No.:BCX0219
CAS No.:2854-40-2
- Demethylmangostanin
Catalog No.:BCX0218
CAS No.:2289591-37-1
- Cyclo(L-Leu-L-Pro)
Catalog No.:BCX0217
CAS No.:2873-36-1
- Apigenin 6-C-(2-O-feruloyl)glucoside 8-C-glucoside
Catalog No.:BCX0230
CAS No.:1287786-63-3
- Fibleucin
Catalog No.:BCX0231
CAS No.:24278-14-6
- N-Acetylnornuciferine
Catalog No.:BCX0232
CAS No.:1942-03-6
- Tinospin E
Catalog No.:BCX0233
CAS No.:1582321-96-7
- Turmeronol B
Catalog No.:BCX0234
CAS No.:131651-38-2
- Aristolodione
Catalog No.:BCX0235
CAS No.:109771-09-7
- Ciwujianoside C2
Catalog No.:BCX0236
CAS No.:114892-56-7
- Mckeanianone B
Catalog No.:BCX0237
CAS No.:2035475-14-8
- 1-O-β-D-Glucopyranosylpaeonisuffrone
Catalog No.:BCX0238
CAS No.:1003888-20-7
- Pteroside B
Catalog No.:BCX0239
CAS No.:29774-74-1
- Kaempferol 3-O-neohesperidoside 7-O-glucoside
Catalog No.:BCX0240
CAS No.:78527-48-7
- Creticoside A
Catalog No.:BCX0241
CAS No.:34336-00-0
Two new alkaloids from the roots of Cocculus hirsutus (L.) W. Theob.[Pubmed:38462768]
Nat Prod Res. 2024 Mar 10:1-12.
Two undescribed alkaloids, 15-carboxydihydroerysotrine (1) and (14 R)-4-methoxy-13,14-dihydrooxypalmatine (2), along with six known compounds, 1,6-didehydro-3,15,16-trimethoxy-9-methylerythrinanium (3), 8-oxytetrahydropalmatine (4), 20-hydroxyecdysone (5), Makisterone A (6) turkesterone (7) and magnoflorine (8) were isolated from the root part of Cocculus hirsutus (L.) W. Theob. Their structures were established based on detailed analysis of NMR, UV-Vis, HRESIMS, and single-crystal XRD spectroscopic experiments. Compounds 3, 4 and 7 were reported for the first time from the genus Cocculus. All the compounds were analysed in silico to investigate their human acetylcholinesterase inhibition potential. This analysis revealed that compounds 1 and 8 interacted well with the selected protein, which suggested their further exploration as acetylcholinesterase inhibitors via in vitro and in vivo investigation.
Identification of Ecdysteroid Sinapate Esters with COX-2 Inhibitory Effects from Fibraurea recisa Using Molecular Networking and MS2LDA.[Pubmed:37530540]
J Nat Prod. 2023 Aug 25;86(8):1960-1967.
The roots of Fibraurea recisa are recognized as a rich source of protoberberine and aporphine alkaloids, but the non-alkaloidal metabolites in this plant are underexplored. The present study investigated the chemical composition of the plant roots using untargeted metabolomics-based molecular networking and MS2LDA motif annotation, revealing the presence of a characteristic fragment motif related to several sinapoyl-functionalized metabolites. Guided by the targeted motif, two new sinapic acid-ecdysteroid hybrids, named 3-O-sinapoyl Makisterone A (1) and 2-O-sinapoyl Makisterone A (2), were isolated. The structures of these compounds, including their absolute configuration, were elucidated by HR-ESIQTOFMS, MS(2) fragmentation, NMR spectroscopy, and chemical degradation coupled with optical rotation measurements. Although neither compound inhibited nitric oxide (NO) production or inducible nitric oxide synthase (iNOS) protein expression on lipopolysaccharide-induced RAW 264 cells, 2 significantly suppressed cyclooxygenase 2 (COX-2) protein expression at 1-30 muM. Additionally, decreased expression of COX-2 protein was barely observed after treatment with methyl sinapate or Makisterone A, the steroid skeleton of 1 and 2. These results indicated that the presence of the sinapoyl moiety at C-2 on the C(28)-ecdysteroid skeleton played a key role in the selectivity for the suppression of the COX-2 protein expression.
Makisterone A attenuates experimental cholestasis by activating the farnesoid X receptor.[Pubmed:35921707]
Biochem Biophys Res Commun. 2022 Oct 1;623:162-169.
Cholestasis is the accumulation of bile acids in the liver due to impaired bile formation, secretion, and excretion caused by infections, drugs, metabolic or genetic diseases. Ursodeoxycholic acid is the only drug approved by the Food and Drug Administration for the treatment of primary biliary cholangitis, but nearly 40% of patients do not adequately respond to this drug and 5-10% show intolerance. The farnesoid X receptor (FXR) plays a key role in bile acid metabolism. Here, by using HERB, a high-throughput experimental and reference-oriented database of herbal medicines, and molecular docking, we identified Makisterone A (MakA) as a compound that could target FXR. We showed that MakA enhanced FXR activity in liver cells and expression levels of FXR target genes in vitro. Importantly, MakA intervention alleviated cholestatic liver injury and dysregulation of hepatic bile acid metabolism induced by alpha-naphthylisothiocyanate and, 5-diethoxycarbonyl-1,4-dihydrocollidine in mice. The ability of MakA to improve liver injury in a mouse model suggests that this drug may be used for clinical treatment of cholestasis.
Fat body lipolysis connects poor nutrition to hypopharyngeal gland degradation in Apis mellifera.[Pubmed:30953617]
J Insect Physiol. 2019 Jul;116:1-9.
The hypopharyngeal glands (HGs) of honey bee nurse workers secrete the major protein fraction of jelly, a protein and lipid rich substance fed to developing larvae, other worker bees, and queens. A hallmark of poorly nourished nurses is their small HGs, which actively degrade due to hormone-induced autophagy. To better connect nutritional stress with HG degradation, we looked to honey bees and other insect systems, where nutrient stress is often accompanied by fat body degradation. The fat body contains stored lipids that are likely a substrate for ecdysteroid synthesis, so we tested whether starvation caused increased fat body lipolysis. Ecdysteroid signaling and response pathways and IIS/TOR are tied to nutrient-dependent autophagy in honey bees and other insects, and so we also tested whether and where genes in these pathways were differentially regulated in the head and fat body. Last, we injected nurse-aged bees with the honey bee ecdysteroid Makisterone A to determine whether this hormone influenced HG size and autophagy. We find that starved nurse aged bees exhibited increased fat body lipolysis and increased expression of ecdysteroid production and response genes in the head. Genes in the IIS/TOR pathway were not impacted by starvation in either the head or fat body. Additionally, bees injected with Makisterone A had smaller HGs and increased expression of autophagy genes. These data support the hypothesis that nutritional stress induces fat body lipolysis, which may liberate the sterols important for ecdysteroid production, and that increased ecdysteroid levels induce autophagic HG degradation.
The ecdysteroidome of Drosophila: influence of diet and development.[Pubmed:26395481]
Development. 2015 Nov 1;142(21):3758-68.
Ecdysteroids are the hormones regulating development, physiology and fertility in arthropods, which synthesize them exclusively from dietary sterols. But how dietary sterol diversity influences the ecdysteroid profile, how animals ensure the production of desired hormones and whether there are functional differences between different ecdysteroids produced in vivo remains unknown. This is because currently there is no analytical technology for unbiased, comprehensive and quantitative assessment of the full complement of endogenous ecdysteroids. We developed a new LC-MS/MS method to screen the entire chemical space of ecdysteroid-related structures and to quantify known and newly discovered hormones and their catabolites. We quantified the ecdysteroidome in Drosophila melanogaster and investigated how the ecdysteroid profile varies with diet and development. We show that Drosophila can produce four different classes of ecdysteroids, which are obligatorily derived from four types of dietary sterol precursors. Drosophila makes Makisterone A from plant sterols and epi-Makisterone A from ergosterol, the major yeast sterol. However, they prefer to selectively utilize scarce ergosterol precursors to make a novel hormone 24,28-dehydroMakisterone A and trace cholesterol to synthesize 20-hydroxyecdysone. Interestingly, epi-Makisterone A supports only larval development, whereas all other ecdysteroids allow full adult development. We suggest that evolutionary pressure against producing epi-C-24 ecdysteroids might explain selective utilization of ergosterol precursors and the puzzling preference for cholesterol.
Ecdysteroids from the flowers of Aerva javanica.[Pubmed:23933119]
Steroids. 2013 Nov;78(11):1098-102.
Four new ecdysteroids (1-4), along with three known steroids, beta-ecdysone (5), 5-beta-2-deoxyintegristerone A (6) and 24-epi-Makisterone A (7) (Fig. 1), were isolated from the methanolic extract of the flowers of Aerva javanica by using normal and reverse phase chromatography. The structures of the new compounds (1-4) were determined due to 1D ((1)H and (13)C), 2D NMR (HSQC, HMBC, COSY, NOESY) techniques and high resolution mass spectrometry (HREIMS). The known compounds (5-7) were characterized based on the 1D NMR spectroscopy and mass spectrometry and by comparison with the literature values. All isolates were evaluated for their inhibitory activities against enzymes acetylcholinesterase (AChE), butyrylcholinesterase (BChE) and lipoxygenase (LOX).
Major constituents and cytotoxic effects of Ajuga chamaecistus ssp. tomentella.[Pubmed:22888532]
Z Naturforsch C J Biosci. 2012 May-Jun;67(5-6):275-81.
The n-butanolic fraction of a methanolic extract (80%) from aerial parts of Ajuga chamaecistus ssp. tomentella was analysed using different chromatographic methods. Column (CC) and high-performance liquid chromatography (HPLC) were used for isolation and purification. 13C, H NMR, H-H COSY, HSQC, HMBC, and ESI-MS were employed for identification of the compounds isolated from this fraction. The structures of the compounds were determined to be cis-melilotoside (1), trans-melilotoside (2), lavandulifolioside (3), 20-hydroxyecdysone (4), leonoside B (5), martynoside (6), ajugalactone (7), Makisterone A (8), and 24-dehydroprecyasterone (9). This is the first report on the presence of cis- and trans-melilotoside in Ajuga species. Cytotoxic evaluation of the n-butanolic fraction, cis- and trans-melilotoside against cancer (T47D, HT-29, and Caco-2) and normal (NIH 3T3) cell lines by the mitochondrial tetrazolium test (MTT) showed no cytotoxic effects up to 400 microg/mL. The results of this study suggest that melilotoside, phenylethyl glycosides, and phytoecdysteroids are the main constituents of the n-butanolic fraction of Ajuga chamaecistus ssp. tomentella.
LC/MS/MS identification of 20-hydroxyecdysone in a scorpion (Liocheles australasiae) and its binding affinity to in vitro-translated molting hormone receptors.[Pubmed:21958716]
Insect Biochem Mol Biol. 2011 Dec;41(12):932-7.
Recent advances in mass spectrometry (MS) technology have facilitated the detection and quantification of minor components in organisms and the environment. In this study, we successfully identified 20-hydroxyecdysone (20E) in first instar nymphs (7 days after hatching) of the scorpion Liocheles australasiae, using tandem mass spectrometry combined with high-performance liquid chromatography (LC/MS/MS). This substance was not found in adults after the fifth stage. Other possible molting hormone candidates such as Makisterone A (MaA) and ponasterone A (PoA), both of which are reported to be the molting hormones of a few arthropod species, were not detected in this scorpion. The ligand-receptor binding of 20E and its analogs was quantitatively evaluated against the in vitro-translated molting hormone receptor, the heterodimer of ecdysone receptor (EcR) and the retinoid X receptor (RXR) of L. australasiae (LaEcR/LaRXR). The concentrations of ecdysone (E), MaA, 20E, and PoA that are required to inhibit 50% of [(3)H]PoA binding to the LaEcR/LaRXR complex were determined to be 1.9, 0.69, 0.05, and 0.017 muM, respectively. The activity profiles of these 4 ecdysteroids are consistent with those obtained for the molting hormone receptors of several insects. The binding of a non-steroidal E agonist, tebufenozide, to EcR was not observed even at high concentrations, indicating that the structure of the ligand-binding pocket of LaEcR is not favorable for interaction with tebufenozide.
Isoforms of the heteropteran Nezara viridula ecdysone receptor: protein characterisation, RH5992 insecticide binding and homology modelling.[Pubmed:21594962]
Pest Manag Sci. 2011 Nov;67(11):1457-67.
BACKGROUND: Certain bisacylhydrazine compounds such as tebufenozide (RH5992) have been shown to act as order-specific insecticides. Their compatibility with predatory Heteroptera, which are used as biological control agents, has also been demonstrated. However, the molecular mode of action of these ecdysone agonists has not been explored in a heteropteran, much less one that is a significant agricultural pest, such as Nezara viridula. RESULTS: Alternatively spliced ligand-binding regions of the N. viridula ecdysone receptor were expressed, purified and characterised by 2D gel analysis, mass spectrometry, homology modelling and competitive binding of a bisacylhydrazine insecticidal compound (RH5992) and various ecdysteroids. Ligand binding by the two splice isoforms was indistinguishable, and relative affinities were found to occur in the order muristerone A > ponasterone A > 20-hydroxyecdysone > inokosterone > RH5992 > alpha-ecdysone. CONCLUSION: The predicted difference in amino acid sequence between the ligand-binding domains of the N. viridula ecdysone receptor splice variants was verified by mass spectrometry. Both splice variant isoforms exhibit a greater affinity for the bisacylhydrazine insecticide RH5992 than do the other hemipteran ecdysone receptors characterised to date. Their affinities for a range of ecdysteroids also distinguish them from the ecdysone receptors of other Hemiptera characterised thus far. Homology models of both N. viridula receptor isoforms provide further insight into the bisacylhydrazine- and ecdysteroid-binding properties of these receptors, including their similar affinity for 20-hydroxyecdysone and the postulated pentatomomorphan moulting hormone Makisterone A.
An ecdysone receptor from the pentatomomorphan, Nezara viridula, shows similar affinities for moulting hormones makisterone A and 20-hydroxyecdysone.[Pubmed:21035548]
Insect Biochem Mol Biol. 2011 Feb;41(2):77-89.
It has been suggested that Pentatomomorpha utilise the C(28) ecdysteroid, Makisterone A (MakA), as the major moulting hormone rather than the more common C(27) hormone, 20-hydroxyecdsyone (20E). The present study is the first to examine this postulate at the level of the ecdysone receptor protein, a heterodimer of nuclear receptors EcR and USP. cDNAs encoding two alternatively spliced isoforms of EcR and a single USP were isolated from a high-quality cDNA library prepared from a representative pentatomomorphan, Nezara viridula (Nv). NvEcR and NvUSP were found to group phylogenetically with heteropteran and other insect EcRs and USP/RXRs, respectively. Sequence comparison and phylogenetic analysis of these proteins found them to be distinct from those belonging to other hemipteran ecdysone receptors characterised to date. Co-expression of the His(6)-tagged ligand binding regions (LBRs) of the two NvEcR variants with the FLAG-tagged LBR of NvUSP was achieved in insect cells employing appropriately constructed baculoviruses. The corresponding heterodimers, designated NvE10 and NvE11, were purified by affinity chromatography utilising the His(6) tags on their NvEcR subunits. The heterodimers displayed nanomolar affinity for [(3)H]ponasterone A (K(d) = 6.8-7.5 nM), characteristic of ecdysone receptors. MakA has a similar affinity to 20E for both NvE10 and NvE11, consistent with MakA being a major moulting hormone in N. viridula.
Dietary effects of four phytoecdysteroids on growth and development of the Indian meal moth, Plodia interpunctella.[Pubmed:20575744]
J Insect Sci. 2010;10:13.
Using pure phytoecdysteroids isolated from Ajuga iva (L.) Schreber (Lamiales: Lamiaceae) and Silene nutans L. (Caryophyllales: Caryophyllaceae), plants known for their high ecdysteroid content, a study was carried out on the effects of ingestion of four different phytoecdysteroids (20-hydroxyecdysone, polypodine B, ponasterone A and Makisterone A) on the growth and development of the Indian meal moth, Plodia interpunctella Hubner (Lepidoptera: Pyralidae) larvae when added at a concentration of 200 ppm in their diet. The experiments clearly showed the susceptibility of P. interpunctella to phytoecdysteroid ingestion. The toxicity of phytoecdysteroids manifested itself by a decrease in larval weight, induction of cannibalism and an increase of mortality, together with disruption of development. The severity of the phytoecdysteroid effect on P. interpunctella depended on the structure of the molecule. The results demonstrate that the minimal structural differences existing between these four phytoecdysteroids significantly affected their toxicity toward P. interpunctella. Makisterone A was the most toxic of the four compounds towards P. interpunctella larvae. In conclusion, phytoecdysteroids ingestion evokes disruptive growth effects on P. interpunctella. This work supports a role for phytoecdysteroids in plant defence against phytophagous insects.


