Cyclo(L-Pro-L-Val)CAS# 2854-40-2 |
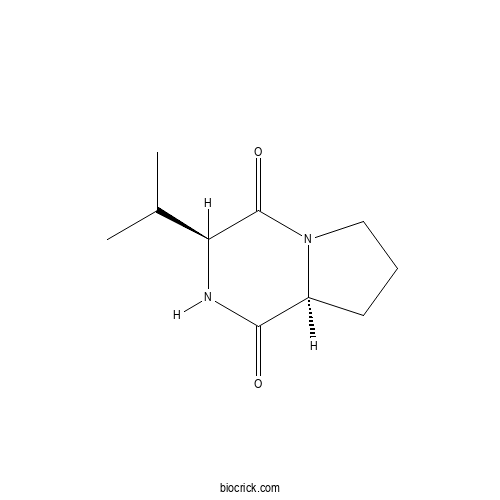
- Cyclo(D-Val-L-Pro)
Catalog No.:BCN4015
CAS No.:27483-18-7
- Cyclo(Pro-Val)
Catalog No.:BCN2420
CAS No.:5654-87-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2854-40-2 | SDF | Download SDF |
| PubChem ID | 6992261 | Appearance | Powder |
| Formula | C10H16N2O2 | M.Wt | 196.25 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3S,8aS)-3-propan-2-yl-2,3,6,7,8,8a-hexahydropyrrolo[1,2-a]pyrazine-1,4-dione | ||
| SMILES | CC(C)C1C(=O)N2CCCC2C(=O)N1 | ||
| Standard InChIKey | XLUAWXQORJEMBD-YUMQZZPRSA-N | ||
| Standard InChI | InChI=1S/C10H16N2O2/c1-6(2)8-10(14)12-5-3-4-7(12)9(13)11-8/h6-8H,3-5H2,1-2H3,(H,11,13)/t7-,8-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Cyclo(L-Pro-L-Val) Dilution Calculator

Cyclo(L-Pro-L-Val) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.0955 mL | 25.4777 mL | 50.9554 mL | 101.9108 mL | 127.3885 mL |
| 5 mM | 1.0191 mL | 5.0955 mL | 10.1911 mL | 20.3822 mL | 25.4777 mL |
| 10 mM | 0.5096 mL | 2.5478 mL | 5.0955 mL | 10.1911 mL | 12.7389 mL |
| 50 mM | 0.1019 mL | 0.5096 mL | 1.0191 mL | 2.0382 mL | 2.5478 mL |
| 100 mM | 0.051 mL | 0.2548 mL | 0.5096 mL | 1.0191 mL | 1.2739 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Demethylmangostanin
Catalog No.:BCX0218
CAS No.:2289591-37-1
- Cyclo(L-Leu-L-Pro)
Catalog No.:BCX0217
CAS No.:2873-36-1
- 25,26,27-Trinor-3α-hydroxycycloartan-24-oic acid
Catalog No.:BCX0216
CAS No.:1300747-31-2
- 3'-Hydroxyliquiritin
Catalog No.:BCX0215
CAS No.:1442113-42-9
- Atalafoline
Catalog No.:BCX0214
CAS No.:59-49-4
- (2R,3S)-Pterosin C
Catalog No.:BCX0213
CAS No.:68399-17-7
- Gnetuhainin O
Catalog No.:BCX0212
CAS No.:337464-95-6
- Pratorimine
Catalog No.:BCX0211
CAS No.:88660-12-2
- Fibraurin
Catalog No.:BCX0210
CAS No.:25254-84-6
- 1-Epideacetylbowdensine
Catalog No.:BCX0209
CAS No.:101219-55-0
- Copalic acid
Catalog No.:BCX0208
CAS No.:24470-48-2
- Diplacone
Catalog No.:BCX0207
CAS No.:73676-38-7
- Cyclo(L-Pro-L-Ile)
Catalog No.:BCX0220
CAS No.:57089-60-8
- Fornicin A
Catalog No.:BCX0221
CAS No.:908588-41-0
- 3β,5α-Dihydroxystigmastan-6-one
Catalog No.:BCX0222
CAS No.:55051-78-0
- 4-(1-Ethoxy-2-hydroxyethyl)benzene-1,2-diol
Catalog No.:BCX0223
CAS No.:1190632-33-7
- Phenacetamide
Catalog No.:BCX0224
CAS No.:103-81-1
- 1,6,8-Trihydroxy-2,7-dimethoxy-3-methylanthraquinone
Catalog No.:BCX0225
CAS No.:2366153-27-5
- 1,3,5,7-Tetrahydroxy-8-prenylxanthone
Catalog No.:BCX0226
CAS No.:444004-76-6
- 22-Epiinotodiol
Catalog No.:BCX0227
CAS No.:64907-49-9
- Brasilixanthone B
Catalog No.:BCX0228
CAS No.:84002-57-3
- Makisterone A
Catalog No.:BCX0229
CAS No.:20137-14-8
- Apigenin 6-C-(2-O-feruloyl)glucoside 8-C-glucoside
Catalog No.:BCX0230
CAS No.:1287786-63-3
- Fibleucin
Catalog No.:BCX0231
CAS No.:24278-14-6
Effect of different storage conditions on the stability and safety of almonds.[Pubmed:36633227]
J Food Sci. 2023 Feb;88(2):848-859.
Almond production in Portugal is of great importance for the economy of their main producing areas. However, the contamination of these nut fruits with fungi and mycotoxins poses a significant risk to food safety and security. This work intended to evaluate the influence of storage conditions on the microbial and mycotoxin stability and safety of almonds throughout long-term storage. Two almond varieties-Lauranne and Guara-were submitted to three different storage conditions, namely, 4 degrees C with noncontrolled relative humidity (RH), 60% RH at 25 degrees C, and 70% RH at 25 degrees C, for a storage period of 9 months. Samples were collected after 0, 3, 6, and 9 months of storage and analyzed for microbial loads (aerobic mesophiles, yeasts, and molds), mold incidence and diversity, and mycotoxin contamination. In total, 26 species were identified belonging to 6 genera: Aspergillus, Cladosporium, Fusarium, Penicillium, Paecilomyces, and Talaromyces. For the variety Guara, mycotoxins related to Aspergillus sect. Flavi, such as aflatoxins, averufin, versicolorin C, and norsolorinic acid, were detected only after 9 months of storage at 70% and 60% RH. Penicillium mycotoxins, such as quinolactacin A and roquefortine C, were also detected. For the variety Lauranne, Penicillium mycotoxins were detected, such as citrinin, quinolactacins A and B, roquefortines C and D, cyclopenin, cyclopenol, penitrem A, viridicatin, and viridicatol. Mycotoxins related to Aspergillus, such as aspulvinone E, flavoglaucin, paspalin, asperglaucide, asperphenamate, cyclo(L-Pro-L-Tyr), and Cyclo(L-Pro-L-Val), were also detected. PRACTICAL APPLICATION: (Optional, for JFS Research Articles ONLY) The quality of almonds depends on the storage period and the RH and temperature at which they are stored. Storage of almonds at 60% RH at 25 degrees C is a good storage condition to maintain the stability and safety of nuts in terms of microbial and mycotoxin contaminations.
Bacterial Lipodepsipeptides and Some of Their Derivatives and Cyclic Dipeptides as Potential Agents for Biocontrol of Pathogenic Bacteria and Fungi of Agrarian Plants.[Pubmed:35395154]
J Agric Food Chem. 2022 Apr 20;70(15):4591-4598.
Biotic stresses (fungi, bacteria, insects, weeds, etc.) are some of the most important causes of the decrease in the quality and quantity of crops that could become an emergency due to a noteworthy increase in the world population. Thus, to overcome these problems, massive use of chemical pesticides has been carried out with heavy consequences for environmental pollution and food safety. An eco-friendly alternative can be using natural compound-based biopesticides with high efficacy and selectivity. Some bacterial lipodepsipeptides (tolaasins I, II, A, D, and E and WLIP together with hexacetyl- and tetrahydro-tolaasin I and WLIP methyl ester) and cyclic dipeptides (cyclo(l-Pro-l-Tyr), cyclo(d-Pro-l-Tyr), Cyclo(L-Pro-L-Val), and cyclo(l-Pro-l-Leu)) were assayed against several pathogenic bacteria and fungi of important agrarian plants. Lipodepsipeptides showed strong growth inhibition of all microorganisms tested in the range of 0.1-0.8 mug/mL, while cyclodipeptides, despite preserving this ability, showed a noteworthily reduced antimicrobial activity being active only in the range of 15-900 mug/mL. Among the lipodepsipeptides and cyclic dipeptides assayed, tolaasin d and cyclo(l-Pro-l-Tyr) (also named maculosin-1) appeared to be the most toxic compounds. Some structure-activity relationships of lipodepsipeptides were also discussed along with their practical application as biopesticides in agriculture.
Bioactive Phytochemicals from Mulberry: Potential Anti-Inflammatory Effects in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages.[Pubmed:34360887]
Int J Mol Sci. 2021 Jul 29;22(15):8120.
The fruits of the mulberry tree (Morus alba L.), known as white mulberry, have been consumed in various forms, including tea, beverages, and desserts, worldwide. As part of an ongoing study to discover bioactive compounds from M. alba fruits, the anti-inflammatory effect of compounds from M. alba were evaluated in lipopolysaccharide (LPS)-stimulated mouse RAW 264.7 macrophages. Phytochemical analysis of the ethanol extract of the M. alba fruits led to the isolation of 22 compounds. Among the isolated compounds, to the best of our knowledge, compounds 1, 3, 5, 7, 11, 12, and 14-22 were identified from M. alba fruits for the first time in this study. Inhibitory effects of 22 compounds on the production of the nitric oxide (NO) known as a proinflammatory mediator in LPS-stimulated RAW 264.7 macrophages were evaluated using NO assays. Western blot analysis was performed to evaluate the anti-inflammatory effects of Cyclo(L-Pro-L-Val) (5). We evaluated whether the anti-inflammatory effects of Cyclo(L-Pro-L-Val) (5) following LPS stimulation in RAW 264.7 macrophages occurred because of phosphorylation of IkappaB kinase alpha (IKKalpha), IkappaB kinase beta (IKKbeta), inhibitor of kappa B alpha (IkappaBalpha), nuclear factor kappa B (NF-kappaB) and activations of inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2). Cyclo(L-Pro-L-Val) (5) significantly suppressed phosphorylations of IKKalpha, IKKbeta, IkappaBalpha, and NF-kappaB and activations of iNOS and COX-2 in a concentration-dependent manner. Taken together, these results indicate that Cyclo(L-Pro-L-Val) (5) can be considered a potential therapeutic agent for the treatment of inflammation-associated disorders.
Unveiling the dermatological potential of marine fungal species components: Antioxidant and inhibitory capacities over tyrosinase.[Pubmed:34028084]
Biotechnol Appl Biochem. 2022 Jun;69(3):1252-1266.
Marine fungi are a rich source of biologically active molecules, but a poorly explored bioresource for cosmeceutical products. This study evaluates the phytochemistry, antioxidant, and antityrosinase effects of the organic extracts of marine fungi isolated from various marine environments in India. Out of 35 screened fungal strains, methanol extracts of strains P2, Talaromyces stipitatus, and D4, Aspergillus terreus exhibited antityrosinase activity of 45% and 43%, respectively, at the lowest concentration of 0.5 mg/mL. The highest free radicals scavenging activity of 94% and 97% was observed at 500 mg/mL, respectively, of the same fungal extracts. The total phenolic content ranged from 8.20 to 20.30 mg/g of the dry weight of extract, expressed as gallic acid equivalent. GC-MS analysis of T. stipitatus and A. terreus extract identified seven and 10 major compounds, respectively. Some of the major compounds included azetidine, (3E)-3-[(3,5-dimethoxybenzoyl)hydrazono]-N-isobutyl butanamide, aziridine, and 3-methylcyclopentanone, 1,1-dimethylcyclohexane, cyclopentane carboxylic acid, N-allyl-4,5,6,7-tetrahydro-2-benzothiophene-1-carboxamide, Cyclo(L-Pro-L-Val), and 3-phenylpropionitrile. In conclusion, this study showed abundant fungal resources in Indian marine environments. A correlation between total phenolic contents of the extracts confirmed that phenolic compounds play an important role in antioxidant as well as antityrosinase activity of the marine fungal extracts and can be viewed as new potential antityrosinase and antioxidant resources.
Growth promotion in Arabidopsis thaliana by bacterial cyclodipeptides involves the TOR/S6K pathway activation.[Pubmed:33387853]
J Plant Physiol. 2021 Feb;257:153343.
Cyclodipeptides (CDPs) are the smallest peptidic molecules that can be produced by diverse organisms such as bacteria, fungi, and animals. They have multiple biological effects. In this paper, we examined the CDPs produced by the bacteria Pseudomonas aeruginosa PAO1, which are known as opportunistic pathogens of humans and plants on TARGET OF RAPAMYCIN (TOR) signaling pathways, and regulation of root system architecture. This bacterium produces the bioactive CDPs: cyclo(L-Pro-L-Leu), cyclo(L-Pro-L-Phe), cyclo(L-Pro-L-Tyr), and Cyclo(L-Pro-L-Val). In a previous report, these molecules were found to modulate basic cellular programs not only via auxin mechanisms but also by promoting the phosphorylation of the S6 ribosomal protein kinase (S6K), a downstream substrate of the TOR kinase. In the present work, we found that the inoculation of Arabidopsis plants with P. aeruginosa PAO1, the non-pathogenic P. aeruginosa DeltalasI/Deltarhll strain (JM2), or by direct exposure of plants to CDPs influenced growth and promoted root branching depending upon the treatment imposed, while genetic evidence using Arabidopsis lines with enhanced or decreased TOR levels indicated a critical role of this pathway in the bacterial phytostimulation.
Isolation of 2,5-diketopiperazines from Lysobacter capsici AZ78 with activity against Rhodococcus fascians.[Pubmed:32352330]
Nat Prod Res. 2021 Dec;35(23):4969-4977.
Inhibitory activity of the biocontrol bacterial strain Lysobacter capsici AZ78 is related to the production of cyclo(l-Pro-l-Tyr), a 2,5-diketopiperazine with in vitro and in vivo toxic activity against Phytophthora infestans and Plasmopara viticola. Further investigation of culture filtrate organic extracts showed its ability to produce other 2,5-diketopiperazines. They were isolated and identified by spectroscopic ((1)H NMR and ESIMS) and physic (specific optical rotation) methods as Cyclo(L-Pro-L-Val), cyclo(d-Pro-d-Phe), cyclo(l-Pro-l-Leu), and cyclo(d-Pro-l-Tyr). When tested against the phytopathogenic Gram-positive bacterium Rhodococcus fascians LMG 3605, Cyclo(L-Pro-L-Val) showed a toxic activity similar to chloramphenicol at a comparable concentration. Overall, these data suggest that 2,5-diketopiperazines represent a class of metabolites characterizing the metabolome of L. capsici AZ78. Furthermore, the toxic activity showed by Cyclo(L-Pro-L-Val) against R. fascians LMG 3605 broaden the spectrum activity of 2,5-diketopiperazines against phytopathogenic microorganisms enforcing their potential development as biopesticides.[Figure: see text].
Multiple Fungal Metabolites Including Mycotoxins in Naturally Infected and Fusarium-Inoculated Wheat Samples.[Pubmed:32316403]
Microorganisms. 2020 Apr 17;8(4):578.
In this study, the occurrence of multiple fungal metabolites including mycotoxins was determined in four different winter wheat varieties in a field experiment in Croatia. One group was naturally infected, while the second group was inoculated with a Fusarium graminearum and F. culmorum mixture to simulate a worst-case infection scenario. Data on the multiple fungal metabolites including mycotoxins were acquired with liquid chromatography with mass spectrometry (LC-MS/MS) multi-(myco)toxin method. In total, 36 different fungal metabolites were quantified in this study: the Fusarium mycotoxins deoxynivalenol (DON), DON-3-glucoside (D3G), 3-acetyldeoxynivalenol (3-ADON), culmorin (CULM), 15-hydroxyculmorin, 5-hydroxyculmorin, aurofusarin, rubrofusarin, enniatin (Enn) A, Enn A1, Enn B, Enn B1, Enn B2, Enn B3, fumonisin B1, fumonisin B2, chrysogin, zearalenone (ZEN), moniliformin (MON), nivalenol (NIV), siccanol, equisetin, beauvericin (BEA), and antibiotic Y; the Alternaria mycotoxins alternariol, alternariolmethylether, altersetin, infectopyron, tentoxin, tenuazonic acid; the Aspergillus mycotoxin kojic acid; unspecific metabolites butenolid, brevianamid F, cyclo(L-Pro-L-Tyr), Cyclo(L-Pro-L-Val), and tryptophol. The most abundant mycotoxins in the inoculated and naturally contaminated samples, respectively, were found to occur at the following average concentrations: DON (19,122/1504 microg/kg), CULM (6109/1010 microg/kg), 15-hydroxyculmorin (56,022/1301 microg/kg), 5-hydroxyculmorin (21,219/863 microg/kg), aurofusarin (43,496/1266 microg/kg). Compared to naturally-infected samples, Fusarium inoculations at the flowering stage increased the concentrations of all Fusarium mycotoxins, except enniatins and siccanol in Ficko, the Aspergillus metabolite kojic acid, the Alternaria mycotoxin altersetin, and unspecific metabolites brevianamid F, butenolid, cyclo(L-Pro-L-Tyr), and Cyclo(L-Pro-L-Val). In contrast to these findings, because of possible antagonistic actions, Fusarium inoculation decreased the concentrations of the Alternaria toxins alternariol, alternariolmethylether, infectopyron, tentoxin, tenuazonic acid, as well as the concentration of the nonspecific metabolite tryptophol.


