MRS 1706Potent and selective A2B inverse agonist CAS# 264622-53-9 |
- MTEP hydrochloride
Catalog No.:BCC1780
CAS No.:1186195-60-7
- mGlu2 agonist
Catalog No.:BCC1745
CAS No.:1311385-32-6
- LY341495
Catalog No.:BCC1724
CAS No.:201943-63-7
- CPPHA
Catalog No.:BCC1501
CAS No.:693288-97-0
- Dipraglurant
Catalog No.:BCC1531
CAS No.:872363-17-2
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 264622-53-9 | SDF | Download SDF |
| PubChem ID | 5139184 | Appearance | Powder |
| Formula | C27H29N5O5 | M.Wt | 503.56 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 6.4 mg/mL (12.71 mM; Need ultrasonic and warming) H2O : < 0.1 mg/mL (insoluble) | ||
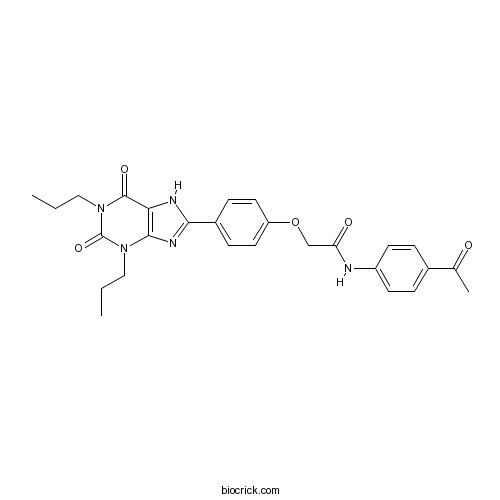
| Chemical Name | N-(4-acetylphenyl)-2-[4-(2,6-dioxo-1,3-dipropyl-7H-purin-8-yl)phenoxy]acetamide | ||
| SMILES | CCCN1C2=C(C(=O)N(C1=O)CCC)NC(=N2)C3=CC=C(C=C3)OCC(=O)NC4=CC=C(C=C4)C(=O)C | ||
| Standard InChIKey | ZKUCFFYOQOJLGT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C27H29N5O5/c1-4-14-31-25-23(26(35)32(15-5-2)27(31)36)29-24(30-25)19-8-12-21(13-9-19)37-16-22(34)28-20-10-6-18(7-11-20)17(3)33/h6-13H,4-5,14-16H2,1-3H3,(H,28,34)(H,29,30) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective adenosine A2B receptor inverse agonist (Ki values are 1.39, 157, 112 and 230 nM for human A2B, A1, A2A and A3 receptors respectively). |

MRS 1706 Dilution Calculator

MRS 1706 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9859 mL | 9.9293 mL | 19.8586 mL | 39.7172 mL | 49.6465 mL |
| 5 mM | 0.3972 mL | 1.9859 mL | 3.9717 mL | 7.9434 mL | 9.9293 mL |
| 10 mM | 0.1986 mL | 0.9929 mL | 1.9859 mL | 3.9717 mL | 4.9647 mL |
| 50 mM | 0.0397 mL | 0.1986 mL | 0.3972 mL | 0.7943 mL | 0.9929 mL |
| 100 mM | 0.0199 mL | 0.0993 mL | 0.1986 mL | 0.3972 mL | 0.4965 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- SSR 146977 hydrochloride
Catalog No.:BCC7635
CAS No.:264618-38-4
- PR-619
Catalog No.:BCC3627
CAS No.:2645-32-1
- Bz-Arg-OEt.HCl
Catalog No.:BCC2686
CAS No.:2645-08-1
- Methyl 2,2-dithienylglycolate
Catalog No.:BCC9034
CAS No.:26447-85-8
- Oxypeucedanin hydrate
Catalog No.:BCN2698
CAS No.:2643-85-8
- 6',7'-Dihydroxybergamottin
Catalog No.:BCN5142
CAS No.:264234-05-1
- Methyl 5-{2-[(tert-butylamino)carbothioyl]carbohydrazonoyl}-1-(2,4-difluorophenyl)-1H-pyrazole-4-carboxylate
Catalog No.:BCC7906
CAS No.:264233-05-8
- SB 415286
Catalog No.:BCC3651
CAS No.:264218-23-7
- Z-Orn-OH
Catalog No.:BCC2757
CAS No.:2640-58-6
- DPPA (Kg)
Catalog No.:BCC2690
CAS No.:26386-88-9
- S 25585
Catalog No.:BCC7687
CAS No.:263849-50-9
- AG 045572
Catalog No.:BCC7464
CAS No.:263847-55-8
- MRS 1754
Catalog No.:BCC7473
CAS No.:264622-58-4
- 26-Deoxyactein
Catalog No.:BCN8076
CAS No.:264624-38-6
- Humulone
Catalog No.:BCN2682
CAS No.:26472-41-3
- Catharanthine Tartrate
Catalog No.:BCN2462
CAS No.:2648-21-5
- 6-Deoxyisojacareubin
Catalog No.:BCN7723
CAS No.:26486-92-0
- Cyclo(D-Phe-L-Pro)
Catalog No.:BCN4011
CAS No.:26488-24-4
- Clovanediol
Catalog No.:BCN5143
CAS No.:2649-64-1
- Clovanediol diacetate
Catalog No.:BCN5144
CAS No.:2649-68-5
- Nandrolone laurate
Catalog No.:BCC9088
CAS No.:26490-31-3
- Z-Gln-OH
Catalog No.:BCC2783
CAS No.:2650-64-8
- Methyleugenolglycol
Catalog No.:BCN6562
CAS No.:26509-45-5
- N-[Bis(methylthio)methylene]- p-toluenesulfonamide
Catalog No.:BCC9069
CAS No.:2651-15-2
Adenosine A2A and A2B Receptor Substantially Attenuate Ischemia/Reperfusion Injury in Septic rat Hearts.[Pubmed:27757725]
Cardiovasc Drugs Ther. 2016 Dec;30(6):551-558.
INTRODUCTION: Mechanical and morphological ischemia and reperfusion (I/R) injury is reduced in septic hearts. The mechanism behind this "cardioprotection" is less well understood. As adenosine receptors play a major role for cardioprotection in non-septic hearts, we investigated the influence of adenosine receptors in a model of I/R in septic hearts. METHODS: SHAM operation or cecal ligation and puncture (CLP) was performed in adult male Wistar rats (n = 60). After 24 h of incubation, hearts were isolated and randomly assigned to a group with or without adenosine receptor (Ador) antagonists (SCH 58261 and MRS 1706) administered before reperfusion. Ischemia and reperfusion lasted for 40 min each. Cardiac function of the heart was determined by measuring left ventricular pressure (LVP). RESULTS: Before I/R, CLP hearts showed a significant mechanical left ventricular impairment (CLP: 63 +/- 5 mmHg vs. SHAM: 104 +/- 6 mmHg. After I/R, left ventricular function was significantly reduced in SHAM (24 +/- 32 mmHg), but not in CLP hearts (65 +/- 13 mmHg). mRNA expression for the AdorA2a and AdorA2b was significantly increased in CLP, but not in SHAM hearts. LVP of CLP hearts deteriorated when AdorA2a and AdorA2b were blocked. CONCLUSIONS: The morphological and functional I/R injury in septic animals is less pronounced compared to non-septic animals. By a combined blockade of AdorA2a and AdorA2b this "cardioprotective" effect is nearly abolished in septic hearts. This is the first study showing, that AdorA2a and AdorA2b may play an important role for a reduced functional I/R injury in the septic heart.
Opposite modulation of astroglial proliferation by adenosine 5'-O-(2-thio)-diphosphate and 2-methylthioadenosine-5'-diphosphate: mechanisms involved.[Pubmed:21419195]
Neuroscience. 2011 May 19;182:32-42.
The contribution of P2Y(12,13) receptors to astroglial proliferation was investigated by testing the effects of two agonists with high affinity for these receptors, adenosine 5'-O-(2-thio)-diphosphate (ADPbetaS) and 2-methylthioadenosine-5'-diphosphate (2-MeSADP), in the incorporation of [(3)H]-thymidine. The effect of ATP, an endogenous inducer of astroglial proliferation, was also investigated. ADPbetaS and ATP (0.01-1 mM) increased astroglial proliferation up to 282%, an effect inhibited by the P2Y(1) receptor antagonist MRS 2179 (30 muM). The P2Y(12) receptor antagonists MRS 2395 (10 muM) and AR-C 66096 (10 muM) also reduced ADPbetaS proliferative effect, whereas the effect of ATP was attenuated by the A(2A) and A(2B) receptor antagonists SCH 58261 (30 nM) and MRS 1706 (10 nM), respectively. Studies of the signalling pathway activated showed that ADPbetaS effect was attenuated by pertussis toxin and by inhibition of phopholipase C (PLC), protein kinase C (PKC) and extracellular signal-regulated kinase1/2 (ERK1/2). The effect of ATP was also attenuated by inhibition of protein kinase A (PKA). The agonist 2-MeSADP (0.001-10 muM) had no effect in astroglial proliferation, but at higher concentrations (0.1-1 mM) it inhibited up to 63%, by mechanisms independent of P2Y(1,12,13) receptors activation. It was metabolised into 2-methylthioadenosine (2-MeSADO), the metabolite responsible for inhibition of astroglial proliferation. The effect of 2-MeSADO (0.1 mM) was attenuated by the A(3) receptors antagonist MRS 1523 (10 muM) and by the inhibitor of nucleoside transporters uridine (0.3 mM). 2-MeSADO did not induce apoptosis but increased lactate dehydrogenase release, an indicator of necrotic cell death. Astroglial proliferation induced by ADPbetaS was mediated by P2Y(1) and P2Y(12) receptors, leading to activation of PLC-PKC-ERK1/2 signalling pathway. The ATP proliferative effect was also mediated by PKA, supporting the contribution of the A(2) receptors. 2-MeSADP inhibition of astroglial proliferation depended on its conversion into 2-MeSADO, which activated A(3) receptors, blocked [(3)H]-thymidine uptake by astrocytes and led to cell death.
In vivo adenosine A(2B) receptor desensitization in guinea-pig airway smooth muscle: implications for asthma.[Pubmed:17716655]
Eur J Pharmacol. 2007 Dec 1;575(1-3):149-57.
This study was aimed at characterizing the role of adenosine receptor subtypes in the contractility modulation of guinea-pig airway smooth muscle in normal and pathological settings. In vitro and in vivo experiments were performed by testing selective agonists and antagonists on isolated tracheal smooth muscle preparations and pulmonary inflation pressure, respectively, under normal conditions or following ovalbumin-induced allergic sensitization. In normal and sensitized animals, the adenosine A(2A)/A(2B) receptor agonist, NECA, evoked relaxing responses of isolated tracheal preparations precontracted with histamine, and such an effect was reversed by the adenosine A(2B) antagonist, MRS 1706, in the presence or in the absence of epithelium. The expression of mRNA coding for adenosine A(2B) receptors was demonstrated in tracheal specimens. In vitro desensitization with 100 microM NECA markedly reduced the relaxing effect of the agonist. In vivo NECA or adenosine administration to normal animals inhibited histamine-mediated bronchoconstriction, while these inhibitory effects no longer occurred in sensitized guinea-pigs. Adenosine plasma levels were significantly higher in sensitized than normal animals. In conclusion, our data demonstrate that: (i) adenosine A(2B) receptors are responsible for the relaxing effects of adenosine on guinea-pig airways; (ii) these receptors can undergo rapid adaptive changes that may affect airway smooth muscle responsiveness to adenosine; (iii) ovalbumin-induced sensitization promotes a reversible inactivation of adenosine A(2B) receptors which can be ascribed to homologous desensitization. These findings can be relevant to better understand adenosine functions in airways as well as mechanisms of action of asthma therapies targeting the adenosine system.
A(2b) receptor mediates adenosine inhibition of taurine efflux from pituicytes.[Pubmed:17391106]
Biol Cell. 2007 Aug;99(8):445-54.
BACKGROUND INFORMATION: Recent work suggests that part of the control of vasopressin output is mediated by taurine released from pituicytes, the astroglial cells of the neurohypophysis. Taurine release, in turn, is stimulated by hypotonic conditions and by vasopressin itself. As adenosine is generated from ATP co-released with vasopressin, it appeared important to study its effects on taurine efflux from pituicytes. RESULTS: We measured radioactive efflux from cultured pituicytes and whole neurohypophyses pre-loaded with [(3)H]taurine. Cultured pituicytes were also used to study adenosine-receptor mRNA expression. Taurine efflux elicited by hypotonic shocks is approximately 30-50% smaller in the presence of 10 microM adenosine or 1 microM NECA (5'-N-ethylcarboxamidoadenosine). Both compounds also inhibited basal efflux in a manner that was not immediately reversible. Agonists of the adenosine A1-, A2a- or A3-receptor subtypes have no relevant effect on basal taurine release, and the A1-receptor antagonist DPCPX (8-cyclopentyl-1,3-dipropylxanthine) has no effect on the inhibition of release by NECA. In turn, the A2b-receptor antagonists MRS 1706 {N-(4-acetylphenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl )phenoxy]acetamide} or alloxazine partially reverse the inhibition of basal or hypotonicity-evoked efflux by NECA. Both A1- and A2b-receptor mRNAs are expressed in pituicytes, which is consistent with an A1-receptor-mediated effect on cell morphology and an A2b-receptor-mediated effect on taurine release. Forskolin and dibutyryl cAMP mimic the inhibitory effects of purinergics on basal taurine efflux, and the adenylate cyclase inhibitor DDA (2',5'-dideoxyadenosine) partially reverses the inhibition of the hypotonic response by NECA.Conclusions. Our results suggest that purinergic inhibition of taurine efflux from pituicytes operates through A2b receptors coupled to intracellular cAMP increase. They point to a possible modulation of neurohypophysial hormone output by endogenous adenosine released in either physiological or pathological situations.
Characterization of adenosine receptors in the human bladder carcinoma T24 cell line.[Pubmed:16581066]
Eur J Pharmacol. 2006 Apr 24;536(1-2):28-37.
The molecular and pharmacological properties of adenosine receptors in the T24 human bladder epithelial carcinoma cell line were assessed by Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR), Ca2+ Flux, cAMP production and interleukin-8 measurements. RT-PCR experiments detected the presence of transcripts for the adenosine A1, A2A and A2B receptors but not for the adenosine A3 subtype. Application of specific adenosine receptor ligands resulted in concentration-dependent increases in intracellular calcium ([Ca2+]i) with the following order of potency and EC50 values: 5'-N-Ethylcarboxamidoadenosine (NECA) (1153+/-214)>5'-(N-Cyclopropyl)carboxamidoadenosine (CPCA) (1436+/-186)>adenosine (4823+/-932). This rank order of potency is typical of adenosine A2B receptors. In addition, select adenosine receptor antagonists N-(4-acetylphenyl)-2-[4-(2,3,6,7-tetrahydro-2,6 dioxo-1,3-dipropyl-1H-purin-8-yl)phenoxy]acetamide (MRS 1706), 8-[4-[((4-Cyano[2,6-]-phenyl)carbamoylmethyl)oxy]phenyl]-1,3-di(n-propyl)-xanthin e (MRS 1754), 1,3-Diethyl-8-phenylxanthine (DPCPX), 1,3-Diethyl-8-phenylxanthine (DPX), Alloxazine, 8-(3-Chlorostyryl)caffeine (CSC), and Theophylline blocked the NECA-induced calcium responses. Additionally, NECA, CPCA, and adenosine stimulated cAMP formation with a rank order of potency characteristic of adenosine A2B receptors. The select adenosine A2A antagonist, 5-amino-7-(phenylethyl)-2-(2-furyl)-pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c] pyrimidine (SCH 58261) failed to antagonize the NECA response, whereas the potent and highly selective adenosine A2B antagonists MRS 1754 and MRS 1706 inhibited NECA-stimulated cAMP production. Lastly, NECA-induced interleukin-8 secretion was inhibited by MRS 1754. Taken together, these data indicate that [Ca2+]i accumulation and cAMP production as well as interleukin-8 secretion is mediated through the adenosine A2B receptor in the T24 cell line.
ZM241385, DPCPX, MRS1706 are inverse agonists with different relative intrinsic efficacies on constitutively active mutants of the human adenosine A2B receptor.[Pubmed:17077318]
J Pharmacol Exp Ther. 2007 Feb;320(2):637-45.
The human adenosine A(2B) receptor belongs to class A G protein-coupled receptors (GPCRs). In our previous work, constitutively active mutant (CAM) human adenosine A(2B) receptors were identified from a random mutation bank. In the current study, three known A(2B) receptor antagonists, 4-{2-[7-amino-2-(2-furyl)[1,2,4]triazolo-[2,3-a][1,3,5]triazin-5-yl-amino]ethyl}p henol (ZM241385), 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), and N-(4-acetylphenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl) phenoxy]acetamide (MRS1706) were tested on wild-type and nine CAM A(2B) receptors with different levels of constitutive activity in a yeast growth assay. All three compounds turned out to be inverse agonists for the adenosine A(2B) receptor because they were able to fully reverse the basal activity of four low-level constitutively active A(2B) receptor mutants and to partially reverse the basal activity of three medium-level constitutively active A(2B) receptor mutants. We also discovered two highly constitutively active mutants whose basal activity could not be reversed by any of the three compounds. A two-state receptor model was used to explain the experimental observations; fitting these yielded the following relative intrinsic efficacies for the three inverse agonists ZM241385, DPCPX, and MRS1706: 0.14 +/- 0.03, 0.35 +/- 0.03, and 0.31 +/- 0.02, respectively. Moreover, varying L, the ratio of active versus inactive receptors in this model, from 0.11 for mutant F84L to 999 for two highly constitutively active mutants yielded simulated dose-response curves that mimicked the experimental curves. This study is the first description of inverse agonists for the human adenosine A(2B) receptor. Moreover, the use of receptor mutants with varying levels of constitutive activity enabled us to determine the relative intrinsic efficacy of these inverse agonists.
Anilide derivatives of an 8-phenylxanthine carboxylic congener are highly potent and selective antagonists at human A(2B) adenosine receptors.[Pubmed:10737749]
J Med Chem. 2000 Mar 23;43(6):1165-72.
No highly selective antagonists of the A(2B) adenosine receptor (AR) have been reported; however such antagonists have therapeutic potential as antiasthmatic agents. Here we report the synthesis of potent and selective A(2B) receptor antagonists. The structure-activity relationships (SAR) of 8-phenyl-1, 3-di-(n-propyl)xanthine derivatives in binding to recombinant human A(2B) ARs in HEK-293 cells (HEK-A(2B)) and at other AR subtypes were explored. Various amide derivatives of 8-[4-[[carboxymethyl]oxy]phenyl]-1,3-di-(n-propyl)xanthine, 4a, were synthesized. A comparison of aryl, alkyl, and aralkyl amides demonstrated that simple anilides, particularly those substituted in the para-position with electron-withdrawing groups, such as nitro, cyano, and acetyl, bind selectively to human A(2B) receptors in the range of 1-3 nM. The unsubstituted anilide 12 had a K(i) value at A(2B) receptors of 1.48 nM but was only moderately selective versus human A(1)/A(2A) receptors and nonselective versus rat A(1) receptors. Highly potent and selective A(2B) antagonists were a p-aminoacetophenone derivative 20 (K(i) value 1.39 nM) and ap-cyanoanilide 27 (K(i) value 1.97 nM). Compound 27 was 400-, 245-, and 123-fold selective for human A(2B) receptors versus human A(1)/A(2A)/A(3) receptors, respectively, and 8.5- and 310-fold selective versus rat A(1)/A(2A) receptors, respectively. Substitution of the 1,3-dipropyl groups with 1,3-diethyl offered no disadvantage for selectivity, and high affinities at A(2B) receptors were maintained. Substitution of the p-carboxymethyloxy group of 4a and its amides with acrylic acid decreased affinity at A(2B) receptors while increasing affinity at A(1) receptors. 1, 3-Di(cyclohexylmethyl) groups greatly reduced affinity at ARs, although the p-carboxymethyloxy derivative 9 was moderately selective for A(2B) receptors. Several selective A(2B) antagonists inhibited NECA-stimulated calcium mobilization in HEK-A(2B) cells.


