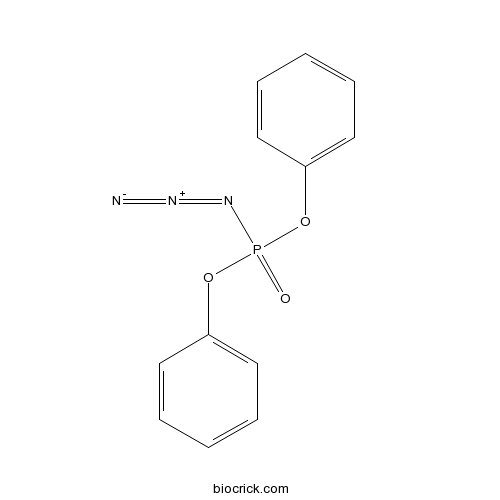
DPPA (Kg)CAS# 26386-88-9 |
- Imatinib Mesylate (STI571)
Catalog No.:BCC1115
CAS No.:220127-57-1
- Dasatinib (BMS-354825)
Catalog No.:BCC1281
CAS No.:302962-49-8
- Saracatinib (AZD0530)
Catalog No.:BCC1166
CAS No.:379231-04-6
- Nilotinib(AMN-107)
Catalog No.:BCC3643
CAS No.:641571-10-0
- Ponatinib (AP24534)
Catalog No.:BCC2522
CAS No.:943319-70-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 26386-88-9 | SDF | Download SDF |
| PubChem ID | 123414 | Appearance | Powder |
| Formula | C12H10N3O3P | M.Wt | 275.2 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [azido(phenoxy)phosphoryl]oxybenzene | ||
| SMILES | C1=CC=C(C=C1)OP(=O)(N=[N+]=[N-])OC2=CC=CC=C2 | ||
| Standard InChIKey | SORGEQQSQGNZFI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H10N3O3P/c13-14-15-19(16,17-11-7-3-1-4-8-11)18-12-9-5-2-6-10-12/h1-10H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

DPPA (Kg) Dilution Calculator

DPPA (Kg) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6337 mL | 18.1686 mL | 36.3372 mL | 72.6744 mL | 90.843 mL |
| 5 mM | 0.7267 mL | 3.6337 mL | 7.2674 mL | 14.5349 mL | 18.1686 mL |
| 10 mM | 0.3634 mL | 1.8169 mL | 3.6337 mL | 7.2674 mL | 9.0843 mL |
| 50 mM | 0.0727 mL | 0.3634 mL | 0.7267 mL | 1.4535 mL | 1.8169 mL |
| 100 mM | 0.0363 mL | 0.1817 mL | 0.3634 mL | 0.7267 mL | 0.9084 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
DPPA (Kg)
- S 25585
Catalog No.:BCC7687
CAS No.:263849-50-9
- AG 045572
Catalog No.:BCC7464
CAS No.:263847-55-8
- 3-Acetoxy-27-hydroxy-20(29)-lupen-28-oic acid methyl ester
Catalog No.:BCN1466
CAS No.:263844-80-0
- 3,27-Dihydroxy-20(29)-lupen-28-oic acid methyl ester
Catalog No.:BCN1467
CAS No.:263844-79-7
- Buergerinin G
Catalog No.:BCN4659
CAS No.:263764-83-6
- EMDT oxalate
Catalog No.:BCC7888
CAS No.:263744-72-5
- PPT
Catalog No.:BCC7062
CAS No.:263717-53-9
- ESI-09
Catalog No.:BCC5504
CAS No.:263707-16-0
- Stachysterone D
Catalog No.:BCC8362
CAS No.:26361-67-1
- H-Lys-OMe .2HCl
Catalog No.:BCC2981
CAS No.:26348-70-9
- Rhodojaponin III
Catalog No.:BCN2809
CAS No.:26342-66-5
- H-Arg-OMe.2HCl
Catalog No.:BCC2861
CAS No.:26340-89-6
- Z-Orn-OH
Catalog No.:BCC2757
CAS No.:2640-58-6
- SB 415286
Catalog No.:BCC3651
CAS No.:264218-23-7
- Methyl 5-{2-[(tert-butylamino)carbothioyl]carbohydrazonoyl}-1-(2,4-difluorophenyl)-1H-pyrazole-4-carboxylate
Catalog No.:BCC7906
CAS No.:264233-05-8
- 6',7'-Dihydroxybergamottin
Catalog No.:BCN5142
CAS No.:264234-05-1
- Oxypeucedanin hydrate
Catalog No.:BCN2698
CAS No.:2643-85-8
- Methyl 2,2-dithienylglycolate
Catalog No.:BCC9034
CAS No.:26447-85-8
- Bz-Arg-OEt.HCl
Catalog No.:BCC2686
CAS No.:2645-08-1
- PR-619
Catalog No.:BCC3627
CAS No.:2645-32-1
- SSR 146977 hydrochloride
Catalog No.:BCC7635
CAS No.:264618-38-4
- MRS 1706
Catalog No.:BCC7120
CAS No.:264622-53-9
- MRS 1754
Catalog No.:BCC7473
CAS No.:264622-58-4
- 26-Deoxyactein
Catalog No.:BCN8076
CAS No.:264624-38-6
Using Diphenylphosphoryl Azide (DPPA) for the Facile Synthesis of Biodegradable Antiseptic Random Copolypeptides.[Pubmed:28169482]
Macromol Rapid Commun. 2017 Apr;38(7).
A facile method has been developed for the large-scale synthesis of random copolypeptides composed of multiple (i.e., cationic, hydrophobic, and hydrophilic) amino acids and their relative ratios have been optimized for broad-spectrum antibacterial effect. The copolypeptides obtained have measured compositions close to the design ratios in spite of the differing reactivities of the different amino acids. An optimized random copolypeptide of lysine, leucine, and serine (denoted as KLS-3) mimicking the composition of LL-37 host defense peptide gives broad spectrum antibacterial activity against clinically relevant Gram-negative and Gram-positive bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa (PAO1) with minimum inhibitory concentrations (MICs) of 32-64 mug mL(-1) , as well as good MICs against multidrug resistant Gram-negative bacteria of Escherichia coli EC 958 (64 mug mL(-1) ) and Klebseilla pneumoniae PTR3 (128 mug mL(-1) ). This method can be applied to the facile large-scale copolymerization of multiple amino acids, including unnatural amino acids, to make effective antibacterial copolypeptides.
Structure of drug delivery DPPA and DPPC liposomes with ligands and their permeability through cells.[Pubmed:24766638]
J Liposome Res. 2015 Mar;25(1):20-31.
Dipalmitoylphosphatidylcholine (DPPC) and 1,2-palmitoyl-phosphatidic acid (DPPA) liposomes, prepared by conventional rotary evaporation method, have similar structural organization, though they have significant differences. The similarity is that both types of lipids create standard bilayer liposomes with strong hydrophobic forces between lipids tails and with homogeneous bonds of hydrogen and electrostatic nature between hydrophilic lipids heads. By the calorimetric method, it has been shown that hydrophobic bonds break but liposomes' destruction does not occur by heating till 150 degrees C. As for bonds between lipid heads in liposomes, their cooperative destruction takes place at 41 degrees C for DPPC and 66 degrees C for DPPA liposomes. In the case of thermal distraction of DPPC liposomes, two so-called pre transitions peaks were observed before the main transition peak, which indicates that DPPC liposomes' structure is multilamellar. DPPA liposomes have one cooperative heat absorption peak, which points to a unilamellar structure of such liposomes. Substances of hydrophobic/hydrophilic nature, incorporated into the liposomes, are placed in hydrophobic or hydrophilic parts of liposomes, which lead to a change in calorimetric peak shapes and thermodynamic parameters. It has been shown that gold nanoparticles, incorporated into the DPPC liposomes, are able to enter Caco-2 cells. In contrast, these nanoparticles do not enter red blood cells.
Organization of collagen in the presence of diphenyl phosphoryl azide (DPPA): an in vitro study.[Pubmed:23624280]
Colloids Surf B Biointerfaces. 2013 Sep 1;109:121-8.
Collagen, an important fibrous protein and its stability is closely related to organizational order. In this work, organization of collagen in the presence of diphenyl phosphoryl azide (DPPA) was studied using circular dichroic spectroscopy, stress-strain characteristics and fibrillogenesis. The reconstituted collagen fibrils in the presence of DPPA were characterized using Fourier transform infrared spectroscopy, differential scanning calorimetry, X-ray diffraction and polarizing light microscopy. CD spectra show that the secondary structure of the collagen molecule is preserved when the concentrations of DPPA is less than 0.018 muM. The Increase in shearing stress with shearing speed is 5-8% higher in the presence of DPPA may be due to the rigidity of the collagen chains. DPPA facilitates self assembly processes, thinner fibrils are seen in polarizing light microscopy and seem to favor the molecular and phase structure of collagen. Thermal stability of collagen in the presence of DPPA ensured the integrity and stabilization of reconstituted collagen fibrillar matrices. Collagen fibrils have higher denaturation enthalpy 15 J/gm at 0.5 muM (DPPA) when compared to 10.5 J/gm for native collagen fibrils which is an indication of more stable fibrils. As a result, the reconstituted collagen fibrils in the presence of DPPA brought about the stabilization of the secondary structure of collagen molecules at lower concentrations of DPPA.
Mono- and polynuclear Ag(i) complexes of N-functionalized bis(diphenylphosphino)amine DPPA-type ligands: synthesis, solid-state structures and reactivity.[Pubmed:28327749]
Dalton Trans. 2017 May 2;46(17):5571-5586.
The reactivity of the N-functionalized DPPA-type ligands (Ph2P)2N(p-Z)C6H4 [Z = H (1a), SMe (1b), OMe (1c)] with AgBF4 was investigated and revealed an unexpected influence of the para substituent Z of the N-aryl ligand. In acetone, the mononuclear bis-chelated [Ag{(1a-1c)-P,P}2]BF4 (2a.BF4-2c.BF4) and dinuclear bridged [Ag2{mu2-(1a-1c)-P,P}2](BF4)2 [3a.(BF4)2-3c.(BF4)2] complexes were obtained with a 1 : 2 and 1 : 1 AgBF4/ligand molar ratio, respectively. While the molecular structures of 2a.BF4 and 2b.BF4 determined in the solid-state by X-ray diffraction revealed their mononuclear nature and the absence of cation/anion interaction, complexes 3b.(BF4)2 and 3c.(BF4)2 form 2D coordination polymers through intermolecular Ag-S or Ag-O interactions, respectively, involving the N-function of the respective DPPA-type ligand, and display direct interactions between one BF4 anion and both Ag(i) cations. Surprisingly, the equimolar reaction between ligands 1a-1c and AgBF4 in CH2Cl2 led to different proportions of the dinuclear complexes 3a.(BF4)2-3c.(BF4)2 and clusters [Ag3(mu3-Cl)2{mu2-(1b-1c)-P,P}3]BF4 (4b.BF4-4c.BF4), depending on the nature of the para substituent Z of the N-aryl ligand. The trinuclear complexes resulted from C-Cl bond activation of the chlorinated solvent and were characterized by NMR spectroscopy and X-ray diffraction, and could be selectively produced by addition of 2/3 equiv. of [NMe4]Cl to the corresponding dinuclear complexes or by a one-pot procedure involving the correct amount of each reagent. A series of experiments and kinetic NMR investigations were performed to gain further insight into the formation of the trinuclear Ag3Cl2 core clusters.


