Luteinizing Hormone Releasing Hormone (LHRH)acitivator of MMP-2 and MMP-9, selective CAS# 33515-09-2 |
- Parathyroid Hormone (1-34), bovine
Catalog No.:BCC1040
CAS No.:12583-68-5
- Angiotensin 1/2 (1-6)
Catalog No.:BCC1036
CAS No.:47896-63-9
- Parathyroid hormone (1-34) (human)
Catalog No.:BCC1046
CAS No.:52232-67-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 33515-09-2 | SDF | Download SDF |
| PubChem ID | 36523 | Appearance | Powder |
| Formula | C55H75N17O13 | M.Wt | 1182.29 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | GONADORELIN; Luliberin; LHRH;LH-Releasing factor; 33515-09-2; LH-Releasing hormone; Gonadotropin-releasing factor | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
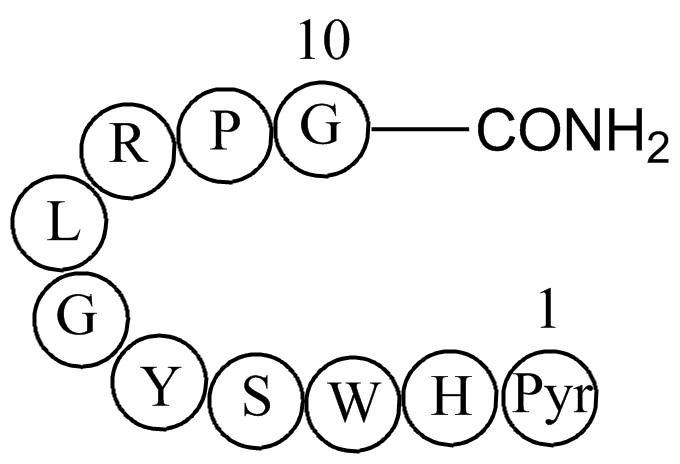
| Sequence | H-DL-Pyr-DL-His-DL-Trp-DL-Ser-DL-Tyr-Gly-DL-Leu-DL-Arg-DL-Pro-Gly-NH2 | ||
| Chemical Name | N-[1-[[1-[[1-[[1-[[2-[[1-[[1-[2-[(2-amino-2-oxoethyl)carbamoyl]pyrrolidin-1-yl]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-2-oxoethyl]amino]-3-(4-hydroxyphenyl)-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-3-(1H-imidazol-5-yl)-1-oxopropan-2-yl]-5-oxopyrrolidine-2-carboxamide | ||
| SMILES | CC(C)CC(C(=O)NC(CCCN=C(N)N)C(=O)N1CCCC1C(=O)NCC(=O)N)NC(=O)CNC(=O)C(CC2=CC=C(C=C2)O)NC(=O)C(CO)NC(=O)C(CC3=CNC4=CC=CC=C43)NC(=O)C(CC5=CN=CN5)NC(=O)C6CCC(=O)N6 | ||
| Standard InChIKey | XLXSAKCOAKORKW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C55H75N17O13/c1-29(2)19-38(49(80)67-37(9-5-17-60-55(57)58)54(85)72-18-6-10-43(72)53(84)62-25-44(56)75)66-46(77)26-63-47(78)39(20-30-11-13-33(74)14-12-30)68-52(83)42(27-73)71-50(81)40(21-31-23-61-35-8-4-3-7-34(31)35)69-51(82)41(22-32-24-59-28-64-32)70-48(79)36-15-16-45(76)65-36/h3-4,7-8,11-14,23-24,28-29,36-43,61,73-74H,5-6,9-10,15-22,25-27H2,1-2H3,(H2,56,75)(H,59,64)(H,62,84)(H,63,78)(H,65,76)(H,66,77)(H,67,80)(H,68,83)(H,69,82)(H,70,79)(H,71,81)(H4,57,58,60) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Glycoprotein hormone involved in the regulation of reproductive processes. |

Luteinizing Hormone Releasing Hormone (LHRH) Dilution Calculator

Luteinizing Hormone Releasing Hormone (LHRH) Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Luteinizing hormone releasing hormone human acetate salt (LHRH) is a selective acitivator of MMP-2 and MMP-9 [1, 2].
Luteinizing hormone-releasing hormone (LHRH), also known as Gonadotropin-releasing hormone (GnRH) is a trophic peptide hormone which secreted by GnRH neurons and plays an important role in the release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the anterior pituitary [3].
MMP-2 (matrix metalloproteinase-2) and MMP-9 (matrix metalloproteinase-9) belong to the MMP family that play an important role in the breakdown of extracellular matrix (ECM) in normal physiological processes, for example, embryonic development, reproduction, and tissue remodeling, as well as in disease processes, such as arthritis and metastasis [2].
When tested with Ishikawa and ECC-1 cell lines, LHRH resulted in the increase of MMP-9 expression which induced cell invasion and it was also showed that GPR101 mediated the LHRH activity and cooperated to function in the metastatic potential of endometrial cancer cells [1]. In human decidual endometrial stromal cells, LHRH with its receptor induced the activation of MMP-2 and MMP-9 [2].
References:
[1]. Cho-Clark, M., et al., GnRH-(1-5) Activates Matrix Metallopeptidase-9 to release Epidermal Growth Factor and Promote Cellular Invasion. Mol Cell Endocrinol, 2015.
[2]. Wu, H.M., et al., Gonadotropin-releasing hormone type II (GnRH-II) agonist regulates the motility of human decidual endometrial stromal cells: possible effect on embryo implantation and pregnancy. Biol Reprod, 2015. 92(4): p. 98.
[3]. Garnick, M.B. and M. Campion, Abarelix Depot, a GnRH antagonist, v LHRH superagonists in prostate cancer: differential effects on follicle-stimulating hormone. Abarelix Depot study group. Mol Urol, 2000. 4(3): p. 275-7.
- Fucoxanthin
Catalog No.:BCN2948
CAS No.:3351-86-8
- Substance P
Catalog No.:BCC6957
CAS No.:33507-63-0
- MEK inhibitor
Catalog No.:BCC1738
CAS No.:334951-92-7
- (S)-HexylHIBO
Catalog No.:BCC7167
CAS No.:334887-48-8
- HexylHIBO
Catalog No.:BCC7166
CAS No.:334887-43-3
- Gisadenafil besylate
Catalog No.:BCC7871
CAS No.:334827-98-4
- Laburnine
Catalog No.:BCN1992
CAS No.:3348-73-0
- Tracheloside
Catalog No.:BCN2738
CAS No.:33464-71-0
- 3-Aminopiperidine dihydrochloride
Catalog No.:BCC8619
CAS No.:334618-23-4
- AH 6809
Catalog No.:BCC1332
CAS No.:33458-93-4
- Evonine
Catalog No.:BCN3087
CAS No.:33458-82-1
- Alnustone
Catalog No.:BCN2761
CAS No.:33457-62-4
- 6'-Iodoresiniferatoxin
Catalog No.:BCC7114
CAS No.:335151-55-8
- Bax channel blocker
Catalog No.:BCC2392
CAS No.:335165-68-9
- iMAC2
Catalog No.:BCC2396
CAS No.:335166-36-4
- Raddeanoside 20
Catalog No.:BCN2796
CAS No.:335354-79-5
- Cyclo(Phe-Leu)
Catalog No.:BCN2418
CAS No.:3354-31-2
- (-)-Bilobalide
Catalog No.:BCN1279
CAS No.:33570-04-6
- Polpunonic acid
Catalog No.:BCN7136
CAS No.:33600-93-0
- Myricanol
Catalog No.:BCN5258
CAS No.:33606-81-4
- Ispinesib (SB-715992)
Catalog No.:BCC2509
CAS No.:336113-53-2
- Britannilactone
Catalog No.:BCN3509
CAS No.:33620-72-3
- Desoxyrhapontigenin
Catalog No.:BCN6479
CAS No.:33626-08-3
- Britannin
Catalog No.:BCN2366
CAS No.:33627-28-0
GnRH antagonists: a new generation of long acting analogues incorporating p-ureido-phenylalanines at positions 5 and 6.[Pubmed:11462984]
J Med Chem. 2001 Feb 1;44(3):453-67.
A series of antagonists of gonadotropin-releasing hormone (GnRH) of the general formula Ac-D2Nal-D4Cpa-D3Pal-Ser-4Aph/4Amf(P)-D4Aph/D4Amf(Q)-Leu-ILys-Pro-DAla-NH2 was synthesized, characterized, and screened for duration of inhibition of luteinizing hormone release in a castrated male rat assay. Selected analogues were tested in a reporter gene assay (IC50 and pA2) and an in vitro histamine release assay. P and Q contain urea/carbamoyl functionalities designed to increase potential intra- and intermolecular hydrogen bonding opportunities for structural stabilization and peptide/receptor interactions, respectively. These substitutions resulted in analogues with increased hydrophilicity and a lesser propensity to form gels in aqueous solution than azaline B [Ac-D2Nal-D4Cpa-D3Pal-Ser-4Aph(Atz)-D4Aph(Atz)-Leu-ILys-Pro-DAla-NH2 with Atz = 3'-amino-1H-1',2',4'-triazol-5'-yl, 5], and in some cases they resulted in a significant increase in duration of action after subcutaneous (s.c.) administration. Ac-D2Nal-D4Cpa-D3Pal-Ser-4Aph(L-hydroorotyl)-D4Aph(carbamoyl)-Leu-ILys-Pro-DAla-N H2 (acetate salt is FE200486) (31) and eight other congeners (20, 35, 37, 39, 41, 45-47) were identified that exhibited significantly longer duration of action than acyline [Ac-D2Nal-D4Cpa-D3Pal-Ser-4Aph(Ac)-D4Aph(Ac)-Leu-ILys-Pro-DAla-NH2] (6) when administered subcutaneously in castrated male rats at a dose of 50 microg in 100 microL of phosphate buffer. No correlation was found between retention times on a C18 reverse phase column using a triethylammonium phosphate buffer at pH 7.0 (a measure of hydrophilicity) or affinity in an in vitro human GnRH report gene assay (pA2) and duration of action. FE200486 was selected for preclinical studies, and some of its properties were compared to those of other clinical candidates. In the intact rat, ganirelix, abarelix, azaline B, and FE200486 inhibited plasma testosterone for 1, 1, 14, and 57 days, respectively, at 2 mg/kg s.c. in 5% mannitol (injection volume = 20 microL). Based on the information that 31, 33, 35 and 37 were significantly shorter acting than acyline or azaline B after intravenous administration (100 microg/rat), we surmised that the very long duration of action of the related FE200486 (for example) was likely due to unique physicochemical properties such as solubility in aqueous milieu, comparatively low propensity to form gels, and ability to diffuse at high concentrations in a manner similar to that described for slow release formulations of peptides. Indeed, in rats injected s.c. with FE200486 (2 mg/kg), plasmatic concentrations of FE200486 remained above 5 ng/mL until day 41, and the time after which they dropped below 3 ng/mL and plasma LH levels started rising until full recovery was reached at day 84 with levels of FE200486 hovering around 1 ng/mL. Additionally, FE200486 was less potent at releasing histamine from isolated rat mast cells than any of the GnRH antagonists presently described in preclinical reports.
Comparison of tolerability and adverse events following treatment with two GnRH agonists in patients with advanced prostate cancer.[Pubmed:24354113]
Urol Nurs. 2013 Sep-Oct;33(5):236-44, 248.
This multicenter, randomized, crossover, open-label study (ClinicalTrials.gov identifier: NCT01161563) assessed patients'and clinicians'perceptions of injection site tolerability and adverse events following the intramuscular injection of triptorelin pamoate or subcutaneous injection of leuprolide acetate in 107 male, patients with advanced prostate cancer.
In vitro effect of gonadotropin-releasing hormone agonist on natural killer cell cytolysis in women with and without endometriosis.[Pubmed:14749633]
Am J Obstet Gynecol. 2004 Jan;190(1):44-9.
OBJECTIVE: The purpose of this study was to assess the in vitro effect of gonadotropin-releasing hormone agonist on natural killer cell activity in women with and without endometriosis and to ascertain whether gonadotropin-releasing hormone agonist effects on natural killer cell activity are direct or mediated solely through the hypoestrogenic state that they produce in vivo. STUDY DESIGN: With use of a chromium 51 release microcytotoxicity assay with K562 target cells, natural killer cell activity was measured after the incubation of mononuclear cells with leuprolide acetate that was obtained from 16 patients with endometriosis and 11 control subjects. RESULTS: The cytotoxicity of natural killer cells that were obtained from patients with endometriosis was reduced significantly (P<.001) with leuprolide. Natural killer cell cytotoxicity from control patients was also significantly decreased (P=.005) with gonadotropin-releasing hormone agonist. Natural killer cell cytotoxicity was significantly lower in patients with endometriosis than in control patients (P=.029). CONCLUSION: These findings suggest a direct immunomodulatory role of gonadotropin-releasing hormone agonist on natural killer cell activity and confirm previous findings that patients with endometriosis have reduced natural killer cell cytotoxicity.
Hydrothermal endometrial ablation can reduce the need for hysterectomy and transfusion.[Pubmed:20932367]
JSLS. 2010 Apr-Jun;14(2):192-5.
Women seeking emergency care for severe uterine hemorrhage with profound anemia often undergo transfusion dilatation curettage and ultimately hysterectomy. The purpose of this article is to describe a modern conservative approach to treating persistent uterine hemorrhage unresponsive to medical therapy, avoiding transfusion and allowing for nonemergent future therapy without the potential complications of transfusion. Six patients with unremitting uterine bleeding were included in the study performed in the Department of Gynecology at an academically affiliated general hospital. Patients underwent successful hydrothermal endometrial ablation after failed medical therapy. This procedure is effective in controlling severe uterine bleeding in patients with large intrauterine fibroids; thus, the number of women being transfused can be significantly reduced.


