Cyclo(Phe-Leu)CAS# 3354-31-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 3354-31-2 | SDF | Download SDF |
| PubChem ID | 562691 | Appearance | Powder |
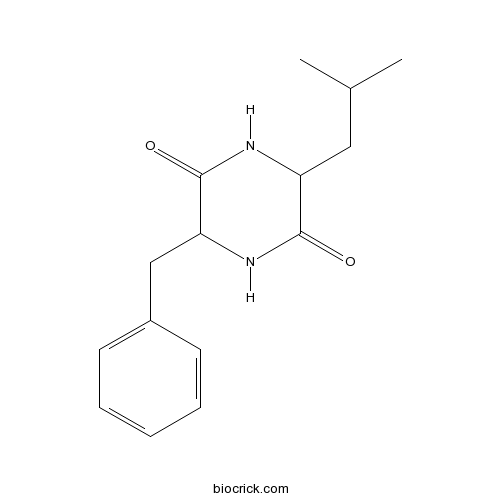
| Formula | C15H20N2O2 | M.Wt | 260.34 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-benzyl-6-(2-methylpropyl)piperazine-2,5-dione | ||
| SMILES | CC(C)CC1C(=O)NC(C(=O)N1)CC2=CC=CC=C2 | ||
| Standard InChIKey | QPDMOMIYLJMOQJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H20N2O2/c1-10(2)8-12-14(18)17-13(15(19)16-12)9-11-6-4-3-5-7-11/h3-7,10,12-13H,8-9H2,1-2H3,(H,16,19)(H,17,18) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cyclo(Phe-Leu) is a new cell cycle inhibitor. |
| In vitro | Cyclic dipeptides as new cell cycle inhibitors produced by Streptomyces flavoretus 18522[Reference: WebLink]Journal of Shenyang Pharmaceutical University, 2015 (2):107 -10.To find the cell cycle inhibitors from the metabolites of Streptomyces flavoretus 18522. |
| Structure Identification | Nat Chem Biol. 2009 Jun;5(6):414-20.Cyclodipeptide synthases are a family of tRNA-dependent peptide bond-forming enzymes.[Pubmed: 19430487]
|

Cyclo(Phe-Leu) Dilution Calculator

Cyclo(Phe-Leu) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.8411 mL | 19.2057 mL | 38.4113 mL | 76.8226 mL | 96.0283 mL |
| 5 mM | 0.7682 mL | 3.8411 mL | 7.6823 mL | 15.3645 mL | 19.2057 mL |
| 10 mM | 0.3841 mL | 1.9206 mL | 3.8411 mL | 7.6823 mL | 9.6028 mL |
| 50 mM | 0.0768 mL | 0.3841 mL | 0.7682 mL | 1.5365 mL | 1.9206 mL |
| 100 mM | 0.0384 mL | 0.1921 mL | 0.3841 mL | 0.7682 mL | 0.9603 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Raddeanoside 20
Catalog No.:BCN2796
CAS No.:335354-79-5
- iMAC2
Catalog No.:BCC2396
CAS No.:335166-36-4
- Bax channel blocker
Catalog No.:BCC2392
CAS No.:335165-68-9
- 6'-Iodoresiniferatoxin
Catalog No.:BCC7114
CAS No.:335151-55-8
- Luteinizing Hormone Releasing Hormone (LHRH)
Catalog No.:BCC1049
CAS No.:33515-09-2
- Fucoxanthin
Catalog No.:BCN2948
CAS No.:3351-86-8
- Substance P
Catalog No.:BCC6957
CAS No.:33507-63-0
- MEK inhibitor
Catalog No.:BCC1738
CAS No.:334951-92-7
- (S)-HexylHIBO
Catalog No.:BCC7167
CAS No.:334887-48-8
- HexylHIBO
Catalog No.:BCC7166
CAS No.:334887-43-3
- Gisadenafil besylate
Catalog No.:BCC7871
CAS No.:334827-98-4
- Laburnine
Catalog No.:BCN1992
CAS No.:3348-73-0
- (-)-Bilobalide
Catalog No.:BCN1279
CAS No.:33570-04-6
- Polpunonic acid
Catalog No.:BCN7136
CAS No.:33600-93-0
- Myricanol
Catalog No.:BCN5258
CAS No.:33606-81-4
- Ispinesib (SB-715992)
Catalog No.:BCC2509
CAS No.:336113-53-2
- Britannilactone
Catalog No.:BCN3509
CAS No.:33620-72-3
- Desoxyrhapontigenin
Catalog No.:BCN6479
CAS No.:33626-08-3
- Britannin
Catalog No.:BCN2366
CAS No.:33627-28-0
- 1-O-Acetylbritannilactone
Catalog No.:BCN7715
CAS No.:33627-41-7
- (S)-(+)-Ketamine hydrochloride
Catalog No.:BCC7930
CAS No.:33643-47-9
- H-Thr-Obzl.HCl
Catalog No.:BCC2674
CAS No.:33645-24-8
- Secnidazole
Catalog No.:BCC4971
CAS No.:3366-95-8
- Hypophyllanthin
Catalog No.:BCN5259
CAS No.:33676-00-5
Cyclodipeptide synthases are a family of tRNA-dependent peptide bond-forming enzymes.[Pubmed:19430487]
Nat Chem Biol. 2009 Jun;5(6):414-20.
Cyclodipeptides and their derivatives belong to the diketopiperazine (DKP) family, which is comprised of a broad array of natural products that exhibit useful biological properties. In the few known DKP biosynthetic pathways, nonribosomal peptide synthetases (NRPSs) are involved in the synthesis of cyclodipeptides that constitute the DKP scaffold, except in the albonoursin (1) pathway. Albonoursin, or cyclo(alpha,beta-dehydroPhe-alpha,beta-dehydroLeu), is an antibacterial DKP produced by Streptomyces noursei. In this pathway, the formation of the Cyclo(Phe-Leu) (2) intermediate is catalyzed by AlbC, a small protein unrelated to NRPSs. We demonstrated that AlbC uses aminoacyl-tRNAs as substrates to catalyze the formation of the DKP peptide bonds. Moreover, several other bacterial proteins, presenting moderate similarity to AlbC, also use aminoacyl-tRNAs to synthesize various cyclodipeptides. Therefore, AlbC and these related proteins belong to a newly defined family of enzymes that we have named cyclodipeptide synthases (CDPSs).


