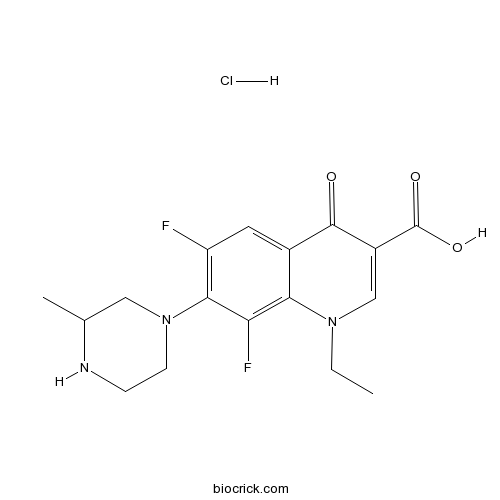
Lomefloxacin HClCAS# 98079-52-8 |
- Apicidin
Catalog No.:BCC3599
CAS No.:183506-66-3
- Scriptaid
Catalog No.:BCC2163
CAS No.:287383-59-9
- Mocetinostat (MGCD0103, MG0103)
Catalog No.:BCC2146
CAS No.:726169-73-9
- PCI-24781 (CRA-024781)
Catalog No.:BCC2155
CAS No.:783355-60-2
- JNJ-26481585
Catalog No.:BCC2147
CAS No.:875320-29-9
- Droxinostat
Catalog No.:BCC2157
CAS No.:99873-43-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 98079-52-8 | SDF | Download SDF |
| PubChem ID | 68624 | Appearance | Powder |
| Formula | C17H20ClF2N3O3 | M.Wt | 387.81 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : 10 mg/mL (25.79 mM; Need ultrasonic and warming) | ||
| Chemical Name | 1-ethyl-6,8-difluoro-7-(3-methylpiperazin-1-yl)-4-oxoquinoline-3-carboxylic acid;hydrochloride | ||
| SMILES | [H+].[Cl-].CCN1C=C(C(O)=O)C(=O)c2cc(F)c(N3CCNC(C)C3)c(F)c12 | ||
| Standard InChIKey | KXEBLAPZMOQCKO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H19F2N3O3.ClH/c1-3-21-8-11(17(24)25)16(23)10-6-12(18)15(13(19)14(10)21)22-5-4-20-9(2)7-22;/h6,8-9,20H,3-5,7H2,1-2H3,(H,24,25);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Lomefloxacin HCl is a fluoroquinolone antibiotic.
Target: Antibacterial
Lomefloxacin is a bactericidal fluoroquinolone agent with activity against a wide range of gram-negative and gram-positive organisms. The bactericidal action of lomefloxacin results from interference with the activity of the bacterial enzymes DNA gyrase and topoisomerase IV, which are needed for the transcription and replication of bacterial DNA. DNA gyrase appears to be the primary quinolone target for gram-negative bacteria. Topoisomerase IV appears to be the preferential target in gram-positive organisms. Interference with these two topoisomerases results in strand breakage of the bacterial chromosome, supercoiling, and resealing. As a result DNA replication and transcription is inhibited [1]. References: | |||||

Lomefloxacin HCl Dilution Calculator

Lomefloxacin HCl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5786 mL | 12.8929 mL | 25.7858 mL | 51.5716 mL | 64.4646 mL |
| 5 mM | 0.5157 mL | 2.5786 mL | 5.1572 mL | 10.3143 mL | 12.8929 mL |
| 10 mM | 0.2579 mL | 1.2893 mL | 2.5786 mL | 5.1572 mL | 6.4465 mL |
| 50 mM | 0.0516 mL | 0.2579 mL | 0.5157 mL | 1.0314 mL | 1.2893 mL |
| 100 mM | 0.0258 mL | 0.1289 mL | 0.2579 mL | 0.5157 mL | 0.6446 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Lomefloxacin HCl is a fluoroquinolone antibiotic.
- Brompheniramine hydrogen maleate
Catalog No.:BCC4515
CAS No.:980-71-2
- Pyrazinamide
Catalog No.:BCC4932
CAS No.:98-96-4
- Nicotinamide
Catalog No.:BCN1025
CAS No.:98-92-0
- Acetophenone
Catalog No.:BCN8300
CAS No.:98-86-2
- H-Pyr-OH
Catalog No.:BCC3328
CAS No.:98-79-3
- Terpineol
Catalog No.:BCN3595
CAS No.:98-55-5
- 4-Aminophenylarsonic acid
Catalog No.:BCC8688
CAS No.:98-50-0
- Benzenesulfonic acid
Catalog No.:BCC8846
CAS No.:98-11-3
- Methyl (E)-3'-hydroxy-4'-methoxycinnamate
Catalog No.:BCN1294
CAS No.:97966-29-5
- Leachianone A
Catalog No.:BCN4530
CAS No.:97938-31-3
- Sophoraflavanone G
Catalog No.:BCN2987
CAS No.:97938-30-2
- Methyl 3-carbazolecarboxylate
Catalog No.:BCN4529
CAS No.:97931-41-4
- Lupinalbin A
Catalog No.:BCN8191
CAS No.:98094-87-2
- Ailanthone
Catalog No.:BCN4531
CAS No.:981-15-7
- Difloxacin HCl
Catalog No.:BCC3764
CAS No.:98106-17-3
- 5,2',6'-Trihydroxy-6,7,8-trimethoxyflavone
Catalog No.:BCN1293
CAS No.:98187-98-5
- Eltoprazine hydrochloride
Catalog No.:BCC5422
CAS No.:98224-03-4
- Eltoprazine
Catalog No.:BCC5421
CAS No.:98206-09-8
- Hupehenine
Catalog No.:BCN2617
CAS No.:98243-57-3
- Isotanshinone II
Catalog No.:BCN3002
CAS No.:98249-39-9
- Boc-D-2-Pal-OH
Catalog No.:BCC2654
CAS No.:98266-32-1
- Boc-D-3-Pal-OH
Catalog No.:BCC2652
CAS No.:98266-33-2
- Nodulisporic acid C2
Catalog No.:BCC8326
CAS No.:
- Finasteride
Catalog No.:BCC2491
CAS No.:98319-26-7
Enhancement of lomefloxacin Hcl ocular efficacy via niosomal encapsulation: in vitro characterization and in vivo evaluation.[Pubmed:27241274]
J Liposome Res. 2017 Dec;27(4):312-323.
The aim of this study is to develop and evaluate niosomal dispersions loaded with the hydrophilic drug; Lomefloxacin HCl (LXN) for the management of ocular bacterial conjunctivitis. LXN-loaded niosomes were prepared by the thin film hydration method following a full factorial formulation design. Two independent variables were evaluated: the type of surfactant (X1) and the surfactant:cholesterol ratio (X2). The dependent variables comprised entrapment efficiency (EE%: Y1), particle size (PS: Y2) and zeta potential (ZP: Y3). The optimum formulation, N-LXN14 (Tw60: CH, 1:1), was spherical in shape and exhibited EE% of 68.41 +/- 0.07, PS of 176.0 +/- 0.98 and ZP of -40.70 +/- 2.20 with a sustained release profile over 8 hours following the Higuchi model. N-LXN14 proved good physicochemical stability under refrigeration up to 3 months. Ocular irritancy test showed no signs of ocular toxicity, confirming the safety and suitability for ocular application. Microbiological evaluation of the antibacterial effect of N-LXN14 was conducted using the susceptibility test and through the induction of topical conjunctivitis by Staphylococcus aureus (S. aureus) followed by topical therapy. Susceptibility test manifested significantly higher percent inhibition of S. aureus and higher AUC0-12 h of N-LXN14 (604.59 +/- 0.05) compared to the commercial product (126.25 +/- 0.049). Both clinical observation and colony count of the infected eyes after eight days of treatment demonstrated significant improvement in therapeutic response. The infected eyes were completely healed with eradication of S. aureus. In conclusion, the results showed that LXN niosomal dispersions may serve as a promising superior ocular delivery system in the treatment of bacterial conjunctivitis.
Design and evaluation of proniosomes as a carrier for ocular delivery of lomefloxacin HCl.[Pubmed:27079800]
J Liposome Res. 2017 Jun;27(2):118-129.
The current investigation aims to develop and evaluate novel ocular proniosomal gels of Lomefloxacin HCl (LXN); in order to improve its ocular bioavailability for the management of bacterial conjunctivitis. Proniosomes were prepared using different types of nonionic surfactants solely and as mixtures with Span 60. The formed gels were characterized for entrapment efficiency, vesicle size, and in vitro drug release. Only Span 60 was able to form stable LXN-proniosomal gel when used individually while the other surfactants formed gels only in combination with Span 60 at different ratios. The optimum proniosomal gel; P-LXN 7 (Span 60:Tween 60, 9:1) appeared as spherical shaped vesicles having high entrapment efficiency (>80%), appropriate vesicle size (187 nm) as well as controlled drug release over 12 h. Differential scanning calorimetry confirmed the amorphous nature of LXN within the vesicles. Stability study did not show any significant changes in entrapment efficiency or vesicle size after storage for 3 months at 4 degrees C. P-LXN 7 was found to be safe and suitable for ocular delivery as proven by the irritancy test. The antibacterial activity of P-LXN 7 evaluated using the susceptibility test and topical therapy of induced ocular conjunctivitis confirmed the enhanced antibacterial therapeutic efficacy of the LXN-proniosomal gel compared to the commercially available LXN eye drops.


