4-Aminophenylarsonic acidCAS# 98-50-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 98-50-0 | SDF | Download SDF |
| PubChem ID | 7389 | Appearance | Powder |
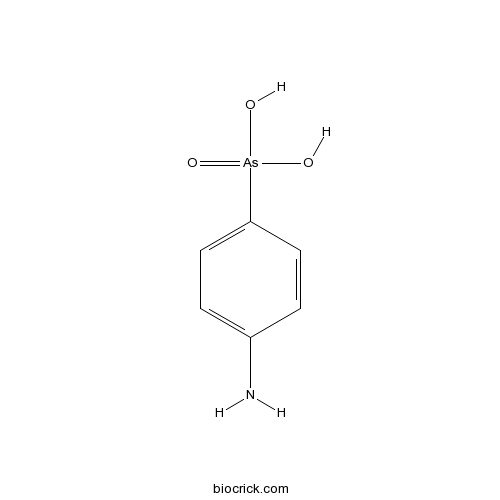
| Formula | C6H8AsNO3 | M.Wt | 217 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (4-aminophenyl)arsonic acid | ||
| SMILES | C1=CC(=CC=C1N)[As](=O)(O)O | ||
| Standard InChIKey | XKNKHVGWJDPIRJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C6H8AsNO3/c8-6-3-1-5(2-4-6)7(9,10)11/h1-4H,8H2,(H2,9,10,11) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

4-Aminophenylarsonic acid Dilution Calculator

4-Aminophenylarsonic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.6083 mL | 23.0415 mL | 46.0829 mL | 92.1659 mL | 115.2074 mL |
| 5 mM | 0.9217 mL | 4.6083 mL | 9.2166 mL | 18.4332 mL | 23.0415 mL |
| 10 mM | 0.4608 mL | 2.3041 mL | 4.6083 mL | 9.2166 mL | 11.5207 mL |
| 50 mM | 0.0922 mL | 0.4608 mL | 0.9217 mL | 1.8433 mL | 2.3041 mL |
| 100 mM | 0.0461 mL | 0.2304 mL | 0.4608 mL | 0.9217 mL | 1.1521 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Benzenesulfonic acid
Catalog No.:BCC8846
CAS No.:98-11-3
- Methyl (E)-3'-hydroxy-4'-methoxycinnamate
Catalog No.:BCN1294
CAS No.:97966-29-5
- Leachianone A
Catalog No.:BCN4530
CAS No.:97938-31-3
- Sophoraflavanone G
Catalog No.:BCN2987
CAS No.:97938-30-2
- Methyl 3-carbazolecarboxylate
Catalog No.:BCN4529
CAS No.:97931-41-4
- 3,4'-Dihydroxy-3,5',7-trimethoxyflavan
Catalog No.:BCN4528
CAS No.:97914-19-7
- Estradiol valerate
Catalog No.:BCC4482
CAS No.:979-32-8
- Norfloxacin lactate
Catalog No.:BCC9104
CAS No.:97867-34-0
- 8,9-Didehydro-7-hydroxydolichodial
Catalog No.:BCN6674
CAS No.:97856-19-4
- Penciclovir Sodium
Catalog No.:BCC5635
CAS No.:97845-62-0
- IMD 0354
Catalog No.:BCC4556
CAS No.:978-62-1
- Lappaconitine Hydrobromide
Catalog No.:BCN2505
CAS No.:97792-45-5
- Terpineol
Catalog No.:BCN3595
CAS No.:98-55-5
- H-Pyr-OH
Catalog No.:BCC3328
CAS No.:98-79-3
- Acetophenone
Catalog No.:BCN8300
CAS No.:98-86-2
- Nicotinamide
Catalog No.:BCN1025
CAS No.:98-92-0
- Pyrazinamide
Catalog No.:BCC4932
CAS No.:98-96-4
- Brompheniramine hydrogen maleate
Catalog No.:BCC4515
CAS No.:980-71-2
- Lomefloxacin HCl
Catalog No.:BCC4673
CAS No.:98079-52-8
- Lupinalbin A
Catalog No.:BCN8191
CAS No.:98094-87-2
- Ailanthone
Catalog No.:BCN4531
CAS No.:981-15-7
- Difloxacin HCl
Catalog No.:BCC3764
CAS No.:98106-17-3
- 5,2',6'-Trihydroxy-6,7,8-trimethoxyflavone
Catalog No.:BCN1293
CAS No.:98187-98-5
- Eltoprazine hydrochloride
Catalog No.:BCC5422
CAS No.:98224-03-4
A two-dimensional silver(I) coordination polymer constructed from 4-aminophenylarsonate and triphenylphosphane: poly[[(mu(3)-4-aminophenylarsonato-kappa(3)N:O:O)(triphenylphosphane-kappaP)silve r(I)] monohydrate].[Pubmed:25836281]
Acta Crystallogr C Struct Chem. 2015 Apr;71(Pt 4):258-61.
The title compound, {[Ag(C6H7AsNO3)(C18H15P)].H2O}n, has been synthesized from the reaction of 4-Aminophenylarsonic acid with silver nitrate, in aqueous ammonia, with the addition of triphenylphosphane (PPh3). The Ag(I) centre is four-coordinated by one amino N atom, one PPh3 P atom and two arsonate O atoms, forming a severely distorted [AgNPO2] tetrahedron. Two Ag(I)-centred tetrahedra are held together to produce a dinuclear [Ag2O2N2P2] unit by sharing an O-O edge. 4-Aminophenylarsonate (Hapa(-)) adopts a mu3-kappa(3)N:O:O-tridentate coordination mode connecting two dinuclear units, resulting in a neutral [Ag(Hapa)(PPh3)]n layer lying parallel to the (101) plane. The PPh3 ligands are suspended on both sides of the [Ag(Hapa)(PPh3)]n layer, displaying up and down orientations. There is an R2(2)(8) hydrogen-bonded dimer involving two arsonate groups from two Hapa(-) ligands related by a centre of inversion. Additionally, there are hydrogen-bonding interactions involving the solvent water molecules and the arsonate and amine groups of the Hapa(-) ligands, and weak pi-pi stacking interactions within the [Ag(Hapa)(PPh3)]n layer. These two-dimensional layers are further assembled by weak van der Waals interactions to form the final architecture.
The three-dimensional hydrogen-bonded structures in the ammonium and sodium salt hydrates of 4-aminophenylarsonic acid.[Pubmed:25093350]
Acta Crystallogr C Struct Chem. 2014 Aug;70(Pt 8):738-41.
The structures of two hydrated salts of 4-Aminophenylarsonic acid (p-arsanilic acid), namely ammonium 4-aminophenylarsonate monohydrate, NH4(+).C6H7AsNO3(-).H2O, (I), and the one-dimensional coordination polymer catena-poly[[(4-aminophenylarsonato-kappaO)diaquasodium]-mu-aqua], [Na(C6H7AsNO3)(H2O)3]n, (II), have been determined. In the structure of the ammonium salt, (I), the ammonium cations, arsonate anions and water molecules interact through inter-species N-H...O and arsonate and water O-H...O hydrogen bonds, giving the common two-dimensional layers lying parallel to (010). These layers are extended into three dimensions through bridging hydrogen-bonding interactions involving the para-amine group acting both as a donor and an acceptor. In the structure of the sodium salt, (II), the Na(+) cation is coordinated by five O-atom donors, one from a single monodentate arsonate ligand, two from monodentate water molecules and two from bridging water molecules, giving a very distorted square-pyramidal coordination environment. The water bridges generate one-dimensional chains extending along c and extensive interchain O-H...O and N-H...O hydrogen-bonding interactions link these chains, giving an overall three-dimensional structure. The two structures reported here are the first reported examples of salts of p-arsanilic acid.
Sensitive determination of phenylarsenic compounds based on a dual preconcentration method with capillary electrophoresis/UV detection.[Pubmed:21683369]
J Chromatogr A. 2011 Jul 22;1218(29):4779-87.
A novel method based on off-line hollow fiber based liquid liquid liquid microextraction (HF-LLLME) combined with on-column anion selective exhaustive injection (ASEI)-capillary electrophoresis/ultraviolet (CE/UV) detection was proposed for the speciation of five phenylarsenic compounds including phenylarsonic acid (PAA), 4-Aminophenylarsonic acid (4-APAA), 4-hydroxyphenylarsonic acid (4-HPAA), 4-nitrophenylarsonic acid (4-NPAA) and 3-nitro-4-hydroxyphenylarsonic acid (NHPAA) in this paper. In HF-LLLME, the target analytes were extracted from 5 mL aqueous samples (donor solution pH 2.15) through a thin phase of tributyl phosphate (TBP) inside the pores of a polypropylene hollow fiber and finally into an 18 muL 0.8 mmol/L Tris acceptor solution inside the lumen of the hollow fiber. Following HF-LLLME, the acceptor solutions were directly analyzed by ASEI-CE/UV. For ASEI, a large plug of water (91% length of total capillary) was introduced into the separation capillary before sample injection in order to prolong the sample injection time, and thus enhance the stacking efficiency. Under the optimized ASEI conditions, up to 236-fold of enrichment factor (EF) was obtained for the ASEI-CE/UV determination of target phenylarsenic compounds. By combining HF-LLLME with ASEI-CE/UV, EFs ranging from 155 to 1780-fold were achieved and the limits of detection (LODs) (at a signal-to-noise ratio of 3) were in the range of 0.68-6.90 mug/L for five phenylarsenic compounds; the relative standard deviations (RSDs) of corrected peak area were 5.6-11.8%. The proposed HF-LLLME-ASEI-CE/UV method was applied for the determination of five target phenylarsenic compounds in pig feed from a local pig farm, and storage pig litter, soil in agricultural field and lake water collected near this pig farm, the recoveries for the spiked samples were in the range of 85.7-104.5%, 66.7-96.2%, 28.9-46.9% and 86.9-107.8% for pig feed, pig litter, soil and lake water, respectively.
Methanogenic inhibition by roxarsone (4-hydroxy-3-nitrophenylarsonic acid) and related aromatic arsenic compounds.[Pubmed:19889499]
J Hazard Mater. 2010 Mar 15;175(1-3):352-8.
Roxarsone (4-hydroxy-3-nitro-phenylarsonic acid) and p-arsanilic acid (4-Aminophenylarsonic acid) are feed additives widely used in the broiler and swine industry. This study evaluated the inhibitory effect of roxarsone, p-arsanilic, and other phenylarsonic compounds on the activity of acetate- and H(2)-utilizing methanogenic microorganisms. Roxarsone, p-arsanilic, and 4-hydroxy-3-aminophenylarsonic acid (HAPA) inhibited acetoclastic and hydrogenotrophic methanogens when supplemented at concentrations of 1mM, and their inhibitory effect increased sharply with incubation time. Phenylarsonic acid (1mM) inhibited acetoclastic but not H(2)-utilizing methanogens. HAPA, a metabolite from the anaerobic biodegradation of roxarsone, was found to be sensitive to autooxidation by oxygen. The compound (2.6mM) caused low methanogenic inhibition (only 14.2%) in short-term assays of 12h when autooxidation was prevented by supplementing HAPA solutions with ascorbate. However, ascorbate-free HAPA solutions underwent spontaneous autooxidation in the presence of oxygen, leading to the formation of highly inhibitory compounds. These results confirm the microbial toxicity of organoarsenic compounds, and they indicate that biotic as well as abiotic transformations can potentially impact the fate and microbial toxicity of these contaminants in the environment.
Anaerobic biotransformation of roxarsone and related N-substituted phenylarsonic acids.[Pubmed:16719096]
Environ Sci Technol. 2006 May 1;40(9):2951-7.
Large quantities of arsenic are introduced into the environment through land application of poultry litter containing the organoarsenical feed additive roxarsone (3-nitro-4-hydroxyphenylarsonic acid). The objective of this study was to evaluate the bioconversion of roxarsone and related N-substituted phenylarsonic acid derivatives under anaerobic conditions. The results demonstrate that roxarsone is rapidly transformed in the absence of oxygen to the corresponding aromatic amine, 4-hydroxy-3-aminophenylarsonic acid (HAPA). The formation of HAPA is attributable to the facile reduction of the nitro group. Electron-donating substrates, such as hydrogen gas, glucose, and lactate, stimulated the rate of nitro group reduction, indicating a microbial role. During long-term incubations, HAPA and the closely related 4-Aminophenylarsonic acid (4-APA) were slowly biologically eliminated by up to 99% under methanogenic and sulfate-reducing conditions, whereas little or no removal occurred in heat-killed inoculum controls. Arsenite and, to a lesser extent, arsenate were observed as products of the degradation. Freely soluble forms of the inorganic arsenical species accounted for 19-28% of the amino-substituted phenylarsonic acids removed. This constitutes the first report of a biologically catalyzed rupture of the phenylarsonic group under anaerobic conditions.
Trace element speciation in poultry litter.[Pubmed:12708677]
J Environ Qual. 2003 Mar-Apr;32(2):535-40.
Trace elements are added to poultry feed for disease prevention and enhanced feed efficiency. High concentrations are found in poultry litter (PL), which raises concerns regarding trace element loading of soils. Trace metal cation solubility from PL may be enhanced by complexation with dissolved organic carbon (DOC). Mineralization of organo-As compounds may result in more toxic species such as As(III) and As(V). Speciation of these elements in PL leachates should assist in predicting their fate in soil. Elemental concentrations of 40 PL samples from the southeastern USA were determined. Water-soluble extractions (WSE) were fractionated into hydrophobic, anionic, and cationic species with solid-phase extraction columns. Arsenic speciation of seven As species, including the main As poultry feed additives, roxarsone (ROX; 3-nitro-4-hydroxyphenylarsonic acid) and p-arsanilic acid (p-ASA; 4-Aminophenylarsonic acid), was performed by ion chromatography-inductively coupled plasma-mass spectrometry (IC-ICP-MS). Total As concentrations in the litter varied from 1 to 39 mg kg(-1), averaging 16 mg kg(-1). Mean total Cu, Ni, and Zn concentrations were 479, 11, and 373 mg kg(-1), respectively. Copper and Ni were relatively soluble (49 and 41% respectively) while only 6% of Zn was soluble. Arsenic was highly soluble with an average of 71% WSE. Roxarsone was the major As species in 50% of PL samples. However, the presence of As(V) as the major species in 50% of the PL samples indicates that mineralization of ROX had occurred. The high solubility of As from litter and its apparent ready mineralization to inorganic forms coupled with the large quantity of litter that is annually land-applied in the USA suggests a potential detrimental effect on soil and water quality in the long term.
Reduction of liver copper concentration by the organic arsenical, 3-nitro-4-hydroxyphenylarsonic acid.[Pubmed:3988630]
J Anim Sci. 1985 Feb;60(2):440-50.
The interaction between 3-nitro-4-hydroxyphenylarsonic acid (roxarsone) and Cu was studied in a series of experiments with crossbred, broiler-type chicks. A fully fortified corn-soybean meal diet was fed in all assays. While roxarsone caused a marked reduction in liver Cu concentration, arsanilic acid (4-Aminophenylarsonic acid), As2O3 and As2O5 were without effect. When structural analogs of roxarsone were studied, it was found that o-nitrophenol and 3-nitro-4-hydroxybenzoic acid also had no effect on liver Cu concentration in birds fed a high level of Cu. However, liver Co concentration was reduced by the addition of either o-nitrophenol or roxarsone to the diets of birds fed a high level of Co. It was concluded that arsenic per se had no effect on liver Cu accumulation or depletion, but that a chelate was probably formed between Cu or Co and the nitroso and hydroxyl groups of the ring portion of roxarsone. In addition to the reduction in liver Cu deposition, concentrations of Cu in the bile, brain, heart and pancreas of chicks were reduced by the addition of roxarsone to a high-Cu diet. Neither dietary nor intraperitoneally (ip) injected roxarsone had an effect on liver Cu concentration when Cu was injected ip. Therefore, both roxarsone and Cu had to be present in the diet for the liver Cu-lowering effect of roxarsone to be exerted. A further experiment was conducted with growing rats to determine the effect of roxarsone on Cu balance. Feeding roxarsone elevated Cu excretion in the urine but had no effect on Cu excretion in the feces.


