LittorineCAS# 21956-47-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 21956-47-8 | SDF | Download SDF |
| PubChem ID | 443005 | Appearance | White to grey powder |
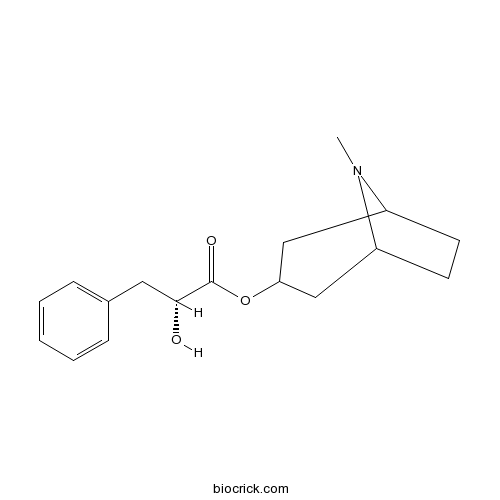
| Formula | C17H23NO3 | M.Wt | 289.37 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in water | ||
| Chemical Name | (8-methyl-8-azabicyclo[3.2.1]octan-3-yl) (2R)-2-hydroxy-3-phenylpropanoate | ||
| SMILES | CN1C2CCC1CC(C2)OC(=O)C(CC3=CC=CC=C3)O | ||
| Standard InChIKey | FNRXUEYLFZLOEZ-LGGPCSOHSA-N | ||
| Standard InChI | InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)11-15(10-13)21-17(20)16(19)9-12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13?,14?,15?,16-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. The kinetics of Littorine biosynthesis were in agreement with the role of this compound as a direct precursor of hyoscyamine biosynthesis. |
| Targets | P450 (e.g. CYP17) |

Littorine Dilution Calculator

Littorine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4558 mL | 17.2789 mL | 34.5578 mL | 69.1157 mL | 86.3946 mL |
| 5 mM | 0.6912 mL | 3.4558 mL | 6.9116 mL | 13.8231 mL | 17.2789 mL |
| 10 mM | 0.3456 mL | 1.7279 mL | 3.4558 mL | 6.9116 mL | 8.6395 mL |
| 50 mM | 0.0691 mL | 0.3456 mL | 0.6912 mL | 1.3823 mL | 1.7279 mL |
| 100 mM | 0.0346 mL | 0.1728 mL | 0.3456 mL | 0.6912 mL | 0.8639 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Boc-Cys(Trt)-OH
Catalog No.:BCC3380
CAS No.:21947-98-8
- trans-Cinnamic anhydride
Catalog No.:BCN7914
CAS No.:21947-71-7
- Boc-Nle-OH.DCHA
Catalog No.:BCC3297
CAS No.:21947-32-0
- H-ß-HoIle-OH.HCl
Catalog No.:BCC3235
CAS No.:219310-10-8
- 2-Hydroxy-3,4,5,6-tetramethoxychalcone
Catalog No.:BCN1489
CAS No.:219298-74-5
- Hydroxyzine 2HCl
Catalog No.:BCC4519
CAS No.:2192-20-3
- Ac-YVAD-AFC
Catalog No.:BCC4022
CAS No.:219137-85-6
- 3'-Methoxydaidzein
Catalog No.:BCN4082
CAS No.:21913-98-4
- 2,3-Dihydro-3-methoxywithaferin A
Catalog No.:BCN7943
CAS No.:21902-96-5
- Boc-ß-HoGlu(OBzl)-OH
Catalog No.:BCC3233
CAS No.:218943-30-7
- Z-Trp(Boc)-OH.DCHA
Catalog No.:BCC2749
CAS No.:218938-57-9
- KNK437
Catalog No.:BCC6399
CAS No.:218924-25-5
- Trans-Pinosylvin dimethyl ether
Catalog No.:BCN3495
CAS No.:21956-56-9
- PD 173074
Catalog No.:BCC3662
CAS No.:219580-11-7
- 4',7-Dihydroxyflavone
Catalog No.:BCN3758
CAS No.:2196-14-7
- beta-Hydroxypropiovanillone
Catalog No.:BCN4938
CAS No.:2196-18-1
- (R)-Coclaurine
Catalog No.:BCN8348
CAS No.:2196-60-3
- Griffipavixanthone
Catalog No.:BCN4939
CAS No.:219649-95-3
- Baicalin
Catalog No.:BCN5901
CAS No.:21967-41-9
- 14-Deoxy-12-hydroxyandrographolide
Catalog No.:BCN4673
CAS No.:219721-33-2
- Taxezopidine L
Catalog No.:BCN6946
CAS No.:219749-76-5
- ANA 12
Catalog No.:BCC6287
CAS No.:219766-25-3
- Bombiprenone
Catalog No.:BCN4940
CAS No.:21978-49-4
- BTB06584
Catalog No.:BCC5106
CAS No.:219793-45-0
Functional genomic analysis of alkaloid biosynthesis in Hyoscyamus niger reveals a cytochrome P450 involved in littorine rearrangement.[Pubmed:16720272]
Chem Biol. 2006 May;13(5):513-20.
Tropane alkaloids are valuable pharmaceutical drugs derived from solanaceous plants such as Hyoscyamus niger (black henbane). The biosynthesis of these molecules, including the nature of the enigmatic rearrangement of (R)-Littorine to (S)-hyoscyamine, is not completely understood. To test the hypothesis that a cytochrome P450 enzyme is involved in this rearrangement, we used virus-induced gene silencing to silence a cytochrome P450, CYP80F1, identified from H. niger roots by EST sequencing. Silencing CYP80F1 resulted in reduced hyoscyamine levels and the accumulation of Littorine. Hyoscyamine was observed in CYP80F1-expressing tobacco hairy roots supplied with (R)-Littorine. Expression in yeast confirmed that CYP80F1 catalyzes the oxidation of (R)-Littorine with rearrangement to form hyoscyamine aldehyde, a putative precursor to hyoscyamine, and without rearrangement to form 3'-hydroxyLittorine. Our data strongly support the involvement of CYP80F1 in the rearrangement of Littorine to hyoscyamine.
Kinetic study of littorine rearrangement in Datura innoxia hairy roots by (13)C NMR spectroscopy.[Pubmed:12193016]
J Nat Prod. 2002 Aug;65(8):1131-5.
The kinetics of tropane alkaloid biosynthesis, particularly the isomerization of Littorine into hyoscyamine, were studied by analyzing the kinetics of carbon-13 ((13)C) in metabolites of Datura innoxia hairy root cultures fed with labeled tropoyl moiety precursors. Both Littorine and hyoscyamine were the major alkaloids accumulated, while scopolamine was never detected. Feeding root cultures with (RS)-phenyl[1,3-(13)C(2)]lactic acid led to (13)C spin-spin coupling detected on C-1' and C-2' of the hyoscyamine skeleton, which validated the intramolecular rearrangement of Littorine into hyoscyamine. Label from phenyl[1-(13)C]alanine or (RS)-phenyl[1,3-(13)C(2)]lactic acid was incorporated at higher levels in Littorine than in hyoscyamine. Initially, the apparent hyoscyamine biosynthesized rate (v(app)()hyo = 0.9 micromol (13)C.flask(-1).d(-1)) was lower than Littorine formation (v(app)()litto = 1.8 micromol (13)C.flask(-1).d(-1)), suggesting that the isomerization reaction could be rate limiting. The results obtained for the kinetics of Littorine biosynthesis were in agreement with the role of this compound as a direct precursor of hyoscyamine biosynthesis.
Norlittorine and norhyoscyamine identified as products of littorine and hyoscyamine metabolism by (13)C-labeling in Datura innoxia hairy roots.[Pubmed:22083085]
Phytochemistry. 2012 Feb;74:105-14.
The presence of two compounds, norLittorine and norhyoscyamine, has been reported in leaves and roots of Datura innoxia; however their metabolic origin in the tropane alkaloid pathway has remained unknown. Precise knowledge of this pathway is a necessary pre-requisite to optimize the production of hyoscyamine and scopolamine in D. innoxia hairy root cultures. The exact structure of norLittorine and norhyoscyamine was confirmed by LC-MS/MS and NMR analyses. Isotopic labeling experiments, using [1-(13)C]-phenylalanine, [1'-(13)C]-Littorine and [1'-(13)C]-hyoscyamine, combined with elicitor treatments, using methyl jasmonate, coronalon and 1-aminocyclopropane-1-carboxylic acid, were used to investigate the metabolic origin of the N-demethylated tropane alkaloids. The results suggest that norLittorine and norhyoscyamine are induced under stress conditions by conversion of Littorine and hyoscyamine. We propose the N-demethylation of tropane alkaloids as a mechanism to detoxify cells in overproducing conditions.
Mechanistic insights into the cytochrome P450-mediated oxidation and rearrangement of littorine in tropane alkaloid biosynthesis.[Pubmed:19693762]
Chembiochem. 2009 Sep 21;10(14):2382-93.
During the biosynthesis of certain tropane alkaloids, Littorine (1) is rearranged to hyoscyamine (3). Recent evidence indicates that this isomerisation is a two-step process in which the first step is an oxidation/rearrangement to give hyoscyamine aldehyde (2). This step is catalysed by CYP80F1, a cytochrome P450 enzyme, which was recently identified from the plant Hyoscyamus niger; CYP80F1 also catalyses the hydroxylation of Littorine at the 3'-position. The mechanisms of the reactions catalysed by CYP80F1 were probed with synthetic deutero and arylfluoro analogues of 1. Measurement of the primary kinetic isotope effects indicates that C3' hydrogen abstraction is the rate-limiting step for the oxidation/rearrangement of natural Littorine, and for the 3'-hydroxylation reaction of the unnatural S enantiomer of Littorine. The character of the intermediates in the oxidation/rearrangement and hydroxylation reaction was probed with the use of arylfluorinated analogues of (R)-Littorine (natural stereoisomer) and (S)-Littorine (unnatural stereoisomer) as substrates for CYP80F1. The relative conversions of ortho-, meta- and para-fluoroLittorine analogues were used to obtain information on the likely intermediacy of either a benzylic radical or benzylic carbocation intermediate. The data suggest that hydroxylation takes place via a benzylic carbocation intermediate, whereas the product profile arising from rearrangement is more consistent with a benzylic radical intermediate.


