3'-MethoxydaidzeinCAS# 21913-98-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 21913-98-4 | SDF | Download SDF |
| PubChem ID | 5319422 | Appearance | Powder |
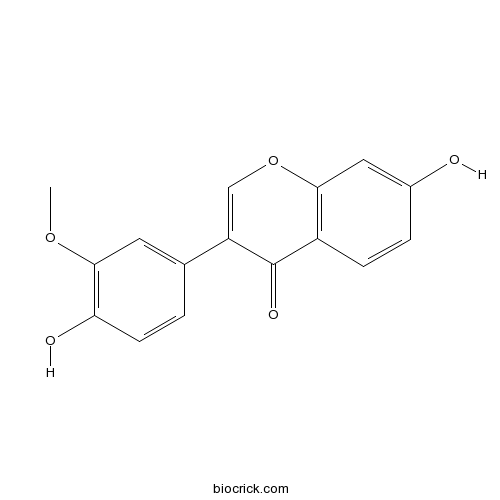
| Formula | C16H12O5 | M.Wt | 284.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 7-hydroxy-3-(4-hydroxy-3-methoxyphenyl)chromen-4-one | ||
| SMILES | COC1=C(C=CC(=C1)C2=COC3=C(C2=O)C=CC(=C3)O)O | ||
| Standard InChIKey | MUYAUELJBWQNDH-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 3'-Methoxydaidzein and geraldol have antiplatelet aggregation activity and appear to be specific against collagen‐induce platelet aggregation with a IC50 values of12.3 and 61.5 uM, respectively. 2. 3'-Methoxydaidzein has antioxidant activity. |
| Targets | PAFR |

3'-Methoxydaidzein Dilution Calculator

3'-Methoxydaidzein Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5174 mL | 17.5871 mL | 35.1741 mL | 70.3482 mL | 87.9353 mL |
| 5 mM | 0.7035 mL | 3.5174 mL | 7.0348 mL | 14.0696 mL | 17.5871 mL |
| 10 mM | 0.3517 mL | 1.7587 mL | 3.5174 mL | 7.0348 mL | 8.7935 mL |
| 50 mM | 0.0703 mL | 0.3517 mL | 0.7035 mL | 1.407 mL | 1.7587 mL |
| 100 mM | 0.0352 mL | 0.1759 mL | 0.3517 mL | 0.7035 mL | 0.8794 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2,3-Dihydro-3-methoxywithaferin A
Catalog No.:BCN7943
CAS No.:21902-96-5
- Boc-ß-HoGlu(OBzl)-OH
Catalog No.:BCC3233
CAS No.:218943-30-7
- Z-Trp(Boc)-OH.DCHA
Catalog No.:BCC2749
CAS No.:218938-57-9
- KNK437
Catalog No.:BCC6399
CAS No.:218924-25-5
- Euphorbia factor L8
Catalog No.:BCN3785
CAS No.:218916-53-1
- 5,15-Diacetyl-3-benzoyllathyrol
Catalog No.:BCN1196
CAS No.:218916-52-0
- Euphorbia factor L2
Catalog No.:BCN3783
CAS No.:218916-51-9
- Ciwujiatone
Catalog No.:BCN7598
CAS No.:218901-26-9
- Taraxeryl acetate
Catalog No.:BCN4937
CAS No.:2189-80-2
- 1-Phenyloctane
Catalog No.:BCN2227
CAS No.:2189-60-8
- Boc-Orn-OH
Catalog No.:BCC3427
CAS No.:21887-64-9
- Horminone
Catalog No.:BCN4936
CAS No.:21887-01-4
- Ac-YVAD-AFC
Catalog No.:BCC4022
CAS No.:219137-85-6
- Hydroxyzine 2HCl
Catalog No.:BCC4519
CAS No.:2192-20-3
- 2-Hydroxy-3,4,5,6-tetramethoxychalcone
Catalog No.:BCN1489
CAS No.:219298-74-5
- H-ß-HoIle-OH.HCl
Catalog No.:BCC3235
CAS No.:219310-10-8
- Boc-Nle-OH.DCHA
Catalog No.:BCC3297
CAS No.:21947-32-0
- trans-Cinnamic anhydride
Catalog No.:BCN7914
CAS No.:21947-71-7
- Boc-Cys(Trt)-OH
Catalog No.:BCC3380
CAS No.:21947-98-8
- Littorine
Catalog No.:BCN1917
CAS No.:21956-47-8
- Trans-Pinosylvin dimethyl ether
Catalog No.:BCN3495
CAS No.:21956-56-9
- PD 173074
Catalog No.:BCC3662
CAS No.:219580-11-7
- 4',7-Dihydroxyflavone
Catalog No.:BCN3758
CAS No.:2196-14-7
- beta-Hydroxypropiovanillone
Catalog No.:BCN4938
CAS No.:2196-18-1
Dual high-resolution alpha-glucosidase and radical scavenging profiling combined with HPLC-HRMS-SPE-NMR for identification of minor and major constituents directly from the crude extract of Pueraria lobata.[Pubmed:25679337]
J Nat Prod. 2015 Feb 27;78(2):294-300.
The crude methanol extract of Pueraria lobata was investigated by dual high-resolution alpha-glucosidase inhibition and radical scavenging profiling combined with hyphenated HPLC-HRMS-SPE-NMR. Direct analysis of the crude extract without preceding purification was facilitated by combining chromatograms from two analytical-scale HPLC separations of 120 and 600 mug on-column, respectively. High-resolution alpha-glucosidase and radical scavenging profiles were obtained after microfractionation of the eluate in 96-well microplates. This allowed full bioactivity profiling of individual peaks in the HPLC chromatogram of the crude methanol extract. Subsequent HPLC-HRMS-SPE-NMR analysis allowed identification of 21 known compounds in addition to two new compounds, i.e., 3'-methoxydaidzein 8-C-[alpha-D-apiofuranosyl-(1-->6)]-beta-D-glucopyranoside and 6''-O-malonyl-3'-methoxydaidzin, as well as an unstable compound tentatively identified as 3'-de-O-methylpuerariafuran.
[Isoflavones from vines of Pueraria lobata].[Pubmed:20353004]
Zhongguo Zhong Yao Za Zhi. 2009 Dec;34(24):3217-20.
OBJECTIVE: To investigate the isoflavones from the vines of Pueraria lobata. METHOD: The compounds were isolated by column chromatography over silica gel and RP-C18, and purified by Sephadex LH-20 column chromatography and preparative TLC. The structures were elucidated on the basis of physico-chemical properties and spectral data. RESULT: Twelve compounds were isolated and identified as: 3'-methoxydaidzein (1), formononetin (2), genistein (3), daidzein (4), daidzin (5), genistin (6), ononin (7), 5-hydroxyl ononin (8), calycosin (9), 6"-O-acetyl genistein (10), 6"-O-acetyl daidzin (11), puerarin (12). CONCLUSION: For the first time, compounds 9-11 were isolated from the genus Pueraria plant, and compounds 1, 3, 6-8 were obtained from the vines of this plant.
Identification of isoflavones in the roots of Pueraria lobata.[Pubmed:17253303]
Planta Med. 1998 Oct;64(7):620-7.
The isoflavones of the roots of Pueraria lobata (Willd.) Ohwi (Puerariae Radix) were investigated by high-performance liquid chromatography (HPLC) coupled to photodiode array (PDA) and to mass spectroscopy (MS) using atmospheric pressure chemical ionization (APCI) or electrospray ionization (ESI) in combination with collision-activated decomposition (CAD) (HPLC-APCI-CAD-MS or ESI-CAD-MS) for identification of glycosides and HPLC-APCI-CAD-MS for identification of aglycones. The major glycosides are derived from daidzein ( 9) and most are 8- C-glycosides. 3'-Hydroxypuerarin-4'- O-deoxyhexoside ( 2B) and 3'-methoxy-6''- O- D-xylosylpuerarin ( 6) were identified as new constituents. MS data were obtained for puerarin-4'- O- D-glucoside ( 1), 3'-hydroxypuerarin ( 2A), puerarin ( 3), 3'-methoxypuerarin ( 4), 6''- O- D-xylosylpuerarin ( 5), daidzin ( 7) and 3'-methoxydaidzin ( 8), which were previously characterized by NMR analysis. Isoflavones identified in Puerariae Radix comprise 3'-methoxydaidzein ( 10), genistein ( 12), daidzein-7- O-methyl ether ( 13A), 3'-methoxydaidzein-7- O-methyl ether or 3'-methoxyformononetin ( 13B) and biochanin A ( 15), while previous characterization of daidzein ( 9) and formononetin ( 14) was substantiated by MS data. The structure of compound 11 could not be established by MS techniques. The estrogenic activity was mainly located in the aglycone fraction.
Activity-guided isolation of constituents of Tephrosia purpurea with the potential to induce the phase II enzyme, quinone reductase.[Pubmed:9322358]
J Nat Prod. 1997 Sep;60(9):869-73.
An isoflavone, 7,4'-dihydroxy-3',5'-dimethoxyisoflavone (1), and a chalcone, (+)-tephropurpurin (2), both novel compounds, as well as six constituents of known structure, (+)-purpurin (3), pongamol (4), lanceolatin B (5), (-)-maackiain (6), (-)-3-hydroxy-4-methoxy-8,9-methylene-dioxypterocarpan (7), and (-)-medicarpin (8), were obtained as active compounds from Tephrosia purpurea, using a bioassay based on the induction of quinone reductase (QR) activity with cultured Hepa 1c1c7 mouse hepatoma cells. Additionally, three inactive compounds of known structure, 3'-methoxydaidzein, desmoxyphyllin B, and 3,9-dihydroxy-8-methoxycoumestan, were isolated and identified. The structure elucidation of compounds 1 and 2 was carried out by spectral data interpretation.


