Leptomycin BCAS# 87081-35-4 |
- Lenalidomide (CC-5013)
Catalog No.:BCC2245
CAS No.:191732-72-6
- Celastrol
Catalog No.:BCN5986
CAS No.:34157-83-0
- Necrostatin 2 racemate
Catalog No.:BCC2077
CAS No.:852391-15-2
- Necrostatin 2
Catalog No.:BCC1793
CAS No.:852391-19-6
- Necrostatin 2 S enantiomer
Catalog No.:BCC2078
CAS No.:852391-20-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 87081-35-4 | SDF | Download SDF |
| PubChem ID | 6436291 | Appearance | Powder |
| Formula | C33H48O6 | M.Wt | 540.74 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in ethanol (supplied pre-dissolved in anhydrous ethanol, 27µg/ml) | ||
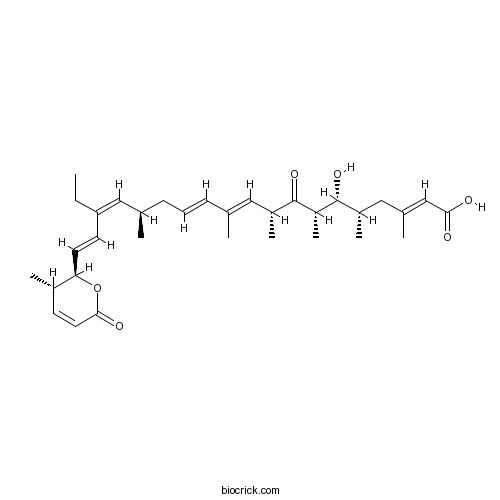
| Chemical Name | (2E,5S,6R,7S,9R,10E,12E,15R,16Z,18E)-17-ethyl-6-hydroxy-3,5,7,9,11,15-hexamethyl-19-[(2R,3S)-3-methyl-6-oxo-2,3-dihydropyran-2-yl]-8-oxononadeca-2,10,12,16,18-pentaenoic acid | ||
| SMILES | CCC(=CC(C)CC=CC(=CC(C)C(=O)C(C)C(C(C)CC(=CC(=O)O)C)O)C)C=CC1C(C=CC(=O)O1)C | ||
| Standard InChIKey | YACHGFWEQXFSBS-NRQIOVFOSA-N | ||
| Standard InChI | InChI=1S/C33H48O6/c1-9-28(14-15-29-24(5)13-16-31(36)39-29)19-22(3)12-10-11-21(2)17-25(6)32(37)27(8)33(38)26(7)18-23(4)20-30(34)35/h10-11,13-17,19-20,22,24-27,29,33,38H,9,12,18H2,1-8H3,(H,34,35)/b11-10+,15-14+,21-17+,23-20+,28-19-/t22-,24+,25-,26+,27-,29-,33-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Antifungal antibiotic that is an inhibitor of the nuclear export of proteins; acts by binding directly to and inhibiting CRM1/exportin-1. Inhibits the nuclear export of the HIV regulatory protein Rev and stabilizes p53. Antitumor in vitro and in vivo. |

Leptomycin B Dilution Calculator

Leptomycin B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8493 mL | 9.2466 mL | 18.4932 mL | 36.9864 mL | 46.2329 mL |
| 5 mM | 0.3699 mL | 1.8493 mL | 3.6986 mL | 7.3973 mL | 9.2466 mL |
| 10 mM | 0.1849 mL | 0.9247 mL | 1.8493 mL | 3.6986 mL | 4.6233 mL |
| 50 mM | 0.037 mL | 0.1849 mL | 0.3699 mL | 0.7397 mL | 0.9247 mL |
| 100 mM | 0.0185 mL | 0.0925 mL | 0.1849 mL | 0.3699 mL | 0.4623 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- AC 261066
Catalog No.:BCC7848
CAS No.:870773-76-5
- PAP-1
Catalog No.:BCC1836
CAS No.:870653-45-5
- Euphohelioscopin A
Catalog No.:BCN6501
CAS No.:87064-61-7
- Zeylasteral
Catalog No.:BCN3065
CAS No.:87064-16-2
- Ritanserin
Catalog No.:BCC7214
CAS No.:87051-43-2
- GW2580
Catalog No.:BCC1096
CAS No.:870483-87-7
- Bis-5,5-nortrachelogenin
Catalog No.:BCN6516
CAS No.:870480-56-1
- 3,23-Dioxo-9,19-cyclolanost-24-en-26-oic acid
Catalog No.:BCN1322
CAS No.:870456-88-5
- CAL-101 (Idelalisib, GS-1101)
Catalog No.:BCC1270
CAS No.:870281-82-6
- Acalisib (GS-9820)
Catalog No.:BCC6384
CAS No.:870281-34-8
- Apilimod mesylate
Catalog No.:BCC5287
CAS No.:870087-36-8
- Tozadenant
Catalog No.:BCC2011
CAS No.:870070-55-6
- MK 0893
Catalog No.:BCC1752
CAS No.:870823-12-4
- E 2012
Catalog No.:BCC1540
CAS No.:870843-42-8
- Mulberrofuran G
Catalog No.:BCN3693
CAS No.:87085-00-5
- 5-Hydroxy-1,7-diphenyl-6-hepten-3-one
Catalog No.:BCN1321
CAS No.:87095-74-7
- Neuromedin B (porcine)
Catalog No.:BCC5841
CAS No.:87096-84-2
- 5-Methoxystrictamine
Catalog No.:BCN4416
CAS No.:870995-64-5
- TAK-285
Catalog No.:BCC3860
CAS No.:871026-44-7
- Raltegravir potassium salt
Catalog No.:BCC5457
CAS No.:871038-72-1
- TCS 3035
Catalog No.:BCC8036
CAS No.:871085-49-3
- Almorexant
Catalog No.:BCC5122
CAS No.:871224-64-5
- Isoneochamaejasmine A
Catalog No.:BCN3131
CAS No.:871319-96-9
- SC 66
Catalog No.:BCC6160
CAS No.:871361-88-5
Leptomycin B ameliorates vasogenic edema formation induced by status epilepticus via inhibiting p38 MAPK/VEGF pathway.[Pubmed:27659963]
Brain Res. 2016 Nov 15;1651:27-35.
The blood-brain barrier (BBB) disruption during brain insults leads to vasogenic edema as one of the primary steps in the epileptogenic process. However, the signaling pathway concerning vasogenic edema formation has not been clarified. In the present study, status epilepticus (SE) resulted in vascular endothelial growth factor (VEGF) over-expression accompanied by loss of BBB integrity in the rat piriform cortex. Leptomycin B (LMB, an inhibitor of chromosome region maintenance 1) attenuated SE-induced vasogenic edema formation. This anti-edema effect of LMB was relevant to inhibitions of VEGF over-expression as well as p38 mitogen-activated protein kinase (MAPK) phosphorylation. Furthermore, SB202190 (a p38 MAPK inhibitor) ameliorated vasogenic edema and VEGF over-expression induced by SE. These findings indicate that p38 MAPK/VEGF signaling pathway may be involved in BBB disruption following SE. Thus, we suggest that p38 MAPK/VEGF axis may be one of therapeutic targets for vasogenic edema in various neurological diseases.
Epigallocatechin-3-gallate enhances the therapeutic effects of leptomycin B on human lung cancer a549 cells.[Pubmed:25922640]
Oxid Med Cell Longev. 2015;2015:217304.
Our previous studies have shown Leptomycin B (LMB) is a promising antilung cancer drug. Epigallocatechin-3-gallate (EGCG) has antitumor properties but a debatable clinical application. The objective of this study is to evaluate the combination therapeutic effect of LMB and EGCG and its molecular mechanisms in human lung cancer A549 cells. Increased cytotoxicity was observed in LMB+EGCG-treated cells compared to LMB-treated cells. Elevated ROS was maximized 2 h after treatment, and LMB+EGCG-treated cells had higher ROS levels compared to LMB. N-Acetyl-L-cysteine (NAC) studies confirmed the oxidative role of LMB and/or EGCG treatment. In comparison to the control, CYP3A4, SOD, GPX1, and p21 mRNA expression levels were increased 7.1-, 2.0-, 4.6-, and 13.1-fold in LMB-treated cells, respectively, while survivin was decreased 42.6-fold. Additionally, these increases of CYP3A4, SOD, and GPX1 were significantly reduced, while p21 was significantly increased in LMB+EGCG-treated cells compared to LMB-treated cells. The qRT-PCR results for p21 and survivin were further confirmed by Western blot. Our study first shows that LMB produces ROS and is possibly metabolized by CYP3A4, GPX1, and SOD in A549 cells, and combination treatment of LMB and EGCG augments LMB-induced cytotoxicity through enhanced ROS production and the modulation of drug metabolism and p21/survivin pathways.
Role of immediate early protein ICP27 in the differential sensitivity of herpes simplex viruses 1 and 2 to leptomycin B.[Pubmed:23740995]
J Virol. 2013 Aug;87(16):8940-51.
Leptomycin B (LMB) is a highly specific inhibitor of CRM1, a cellular karyopherin-beta that transports nuclear export signal-containing proteins from the nucleus to the cytoplasm. Previous work has shown that LMB blocks herpes simplex virus 1 (HSV-1) replication in Vero cells and that certain mutations in viral immediate early protein ICP27 can confer LMB resistance. However, little is known of the molecular mechanisms involved. Here we report that HSV-2, a close relative of HSV-1, is naturally resistant to LMB. To see whether the ICP27 gene determines this phenotypic difference, we generated an HSV-1 mutant that expresses the HSV-2 ICP27 instead of the HSV-1 protein. This recombinant was fully sensitive to LMB, indicating that one or more other viral genes must be important in determining HSV-2's LMB-resistant phenotype. In additional work, we report several findings that shed light on how HSV-1 ICP27 mutations can confer LMB resistance. First, we show that LMB treatment of HSV-1-infected cells leads to suppression of late viral protein synthesis and a block to progeny virion release. Second, we identify a novel type of ICP27 mutation that can confer LMB resistance, that being the addition of a 100-residue amino-terminal affinity purification tag. Third, by studying infections where both LMB-sensitive and LMB-resistant forms of ICP27 are present, we show that HSV-1's sensitivity to LMB is dominant to its resistance. Together, our results suggest a model in which the N-terminal portion of ICP27 mediates a nonessential activity that interferes with HSV-1 replication when CRM1 is inactive. We suggest that LMB resistance mutations weaken or abrogate this activity.
Anti-parasitic effects of Leptomycin B isolated from Streptomyces sp. CJK17 on marine fish ciliate Cryptocaryon irritans.[Pubmed:26827867]
Vet Parasitol. 2016 Feb 15;217:89-94.
The present study was conducted aiming to evaluate the in vitro and in vivo anti-parasitic efficacy of an isolated compound against the ciliate Cryptocaryon irritans. The compound was previously isolated from fermentation products of Streptomyces sp. CJK17 and designated as SFrD. Toxicity of the compound SFrD against the fish hosts (Larimichthys crocea) was also tested and its chemical structure was elucidated. The obtained results showed that the compound has potent anti-parasitic efficacy with the 10 min-, 1 h-, 2 h-, 3 h- and 4 h-LC50 (95% Confidence Intervals) of 6.8 (6.5-7.1), 3.9 (2.8-5.0), 3.3 (2.6-4.0), 2.7 (2.3-3.1) and 2.5 (2.2-2.8) mg L(-1) against theronts of C. irritans and the 6h-LC50 (95% CI) of 3.0 (2.8-3.2) mg L(-1) against the tomonts, respectively. Exposure of the compound SFrD remarkably reduced the mortality of fish infected with C. Irritans, from 100% in the control group to 61.7% and 38.3% in groups of 3.1 mg L(-1) and 6.3 mg L(-1), respectively. In the test of exposing fish to 40 mg L(-1) compound SFrD for 24h, no visible effects were observed affecting the normal behavior or any macroscopic changes. By spectrum analysis (EI-MS, (1)H NMR and (13)C NMR), the compound SFrD was identified as Leptomycin B. This study firstly demonstrated that Leptomycin B has potent anti-parasitic efficacy against ciliates in cultured marine fish.


