LansoprazoleH+,K+-ATPase inhibitor CAS# 103577-45-3 |
- Tenofovir
Catalog No.:BCC2500
CAS No.:147127-20-6
- Tenofovir Disoproxil Fumarate
Catalog No.:BCC1108
CAS No.:202138-50-9
- Entecavir Hydrate
Catalog No.:BCC1109
CAS No.:209216-23-9
- Lersivirine
Catalog No.:BCC1698
CAS No.:473921-12-9
- Didanosine
Catalog No.:BCC3763
CAS No.:69655-05-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 103577-45-3 | SDF | Download SDF |
| PubChem ID | 3883 | Appearance | Powder |
| Formula | C16H14F3N3O2S | M.Wt | 369.36 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Prevacid; Bamalite; Monolitum; Limpidex | ||
| Solubility | DMSO : 100 mg/mL (270.74 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
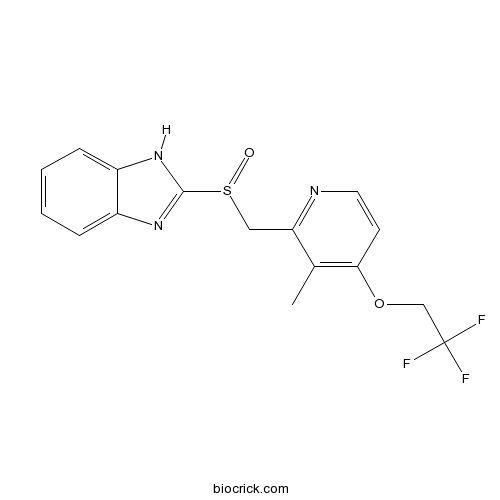
| Chemical Name | 2-[[3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methylsulfinyl]-1H-benzimidazole | ||
| SMILES | CC1=C(C=CN=C1CS(=O)C2=NC3=CC=CC=C3N2)OCC(F)(F)F | ||
| Standard InChIKey | MJIHNNLFOKEZEW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H14F3N3O2S/c1-10-13(20-7-6-14(10)24-9-16(17,18)19)8-25(23)15-21-11-4-2-3-5-12(11)22-15/h2-7H,8-9H2,1H3,(H,21,22) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | H+,K+-ATPase inhibitor (IC50 = 6.3 μM) that displays antisecretory and antiulcer activity. Inhibits gastric acid secretion (IC50 = 0.09 μM for histamine-induced acid formation) and reduces gastric lesion formation induced by a variety of ulcerative stimuli. Antibacterial against Helicobacter pylori in vitro. Also blocks swelling-dependent chloride channel (ICIswell) in NIH3T3 fibroblasts. More potent than omeprazole |

Lansoprazole Dilution Calculator

Lansoprazole Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7074 mL | 13.5369 mL | 27.0739 mL | 54.1477 mL | 67.6846 mL |
| 5 mM | 0.5415 mL | 2.7074 mL | 5.4148 mL | 10.8295 mL | 13.5369 mL |
| 10 mM | 0.2707 mL | 1.3537 mL | 2.7074 mL | 5.4148 mL | 6.7685 mL |
| 50 mM | 0.0541 mL | 0.2707 mL | 0.5415 mL | 1.083 mL | 1.3537 mL |
| 100 mM | 0.0271 mL | 0.1354 mL | 0.2707 mL | 0.5415 mL | 0.6768 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
H+,K+-ATPase inhibitor (IC50 = 6.3 μM) that displays antisecretory and antiulcer activity. Inhibits gastric acid secretion (IC50 = 0.09 μM for histamine-induced acid formation) and reduces gastric lesion formation induced by a variety of ulcerative stimul
- TAK-733
Catalog No.:BCC4587
CAS No.:1035555-63-5
- 3,7-O-Diacetylpinobanksin
Catalog No.:BCN5849
CAS No.:103553-98-6
- Huperzine B
Catalog No.:BCN1059
CAS No.:103548-82-9
- Apiosylskimmin
Catalog No.:BCN2455
CAS No.:103529-94-8
- AZD4547
Catalog No.:BCC3711
CAS No.:1035270-39-3
- AZ505 ditrifluoroacetate
Catalog No.:BCC4265
CAS No.:1035227-44-1
- AZ505
Catalog No.:BCC4264
CAS No.:1035227-43-0
- Phyllanthin
Catalog No.:BCN5848
CAS No.:10351-88-9
- 9-Dehydroandrostenedione
Catalog No.:BCC8801
CAS No.:1035-69-4
- Fmoc-D-N-Me-Leu-OH
Catalog No.:BCC3346
CAS No.:103478-63-3
- Fmoc-N-Me-Leu-OH
Catalog No.:BCC3345
CAS No.:103478-62-2
- Fmoc-D-N- Me-Val-OH
Catalog No.:BCC3359
CAS No.:103478-58-6
- P005672 hydrochloride
Catalog No.:BCC6406
CAS No.:1035979-44-2
- Isookanin
Catalog No.:BCN6476
CAS No.:1036-49-3
- 5,7-Dimethoxyflavanone
Catalog No.:BCN3569
CAS No.:1036-72-2
- RETRA hydrochloride
Catalog No.:BCC2415
CAS No.:1036069-26-7
- Janolusimide
Catalog No.:BCN1840
CAS No.:103612-45-9
- H-Phe(2-Cl)-OH
Catalog No.:BCC3165
CAS No.:103616-89-3
- (±)-5'-Chloro-5'-deoxy-ENBA
Catalog No.:BCC7716
CAS No.:103626-26-2
- Sumatriptan
Catalog No.:BCC5645
CAS No.:103628-46-2
- Sumatriptan Succinate
Catalog No.:BCC2502
CAS No.:103628-48-4
- Cnidimol A
Catalog No.:BCN7167
CAS No.:103629-80-7
- Catechin 3-rhamnoside
Catalog No.:BCN5850
CAS No.:103630-03-1
- Ondansetron hydrochloride dihydrate
Catalog No.:BCC4213
CAS No.:103639-04-9
Formulation and Optimization of Lansoprazole Pellets Using Factorial Design Prepared by Extrusion-Spheronization Technique Using Carboxymethyl Tamarind Kernel Powder.[Pubmed:28088896]
Recent Pat Drug Deliv Formul. 2017;11(1):54-66.
BACKGROUND: In the present study, Lansoprazole pellets were prepared employing a novel excipient Carboxymethyl tamarind kernel powder (CMTKP) using extrusion-spheronization technique. Various research studies including patents have been carried out on this polymer. Pellet formulation was optimized for formulation parameters (concentration of microcrystalline cellulose, CMTKP, croscarmellose sodium and isopropyl alcohol). METHODS: Process parameters (speed and duration of spheronization) were optimized using factorial design. The pellets were evaluated for yield, bulk and tapped density, particle size, hardness, drug content, disintegration time and drug release. RESULTS: The optimized batch showed 93.53% yield, 0.307 kg/cm2 hardness, 2.15 mm average particle size, 292 sec disintegration time and 90.46% drug content. CONCLUSION: Drug release of the optimized batch (2F7) and marketed formulation (LANZOL cap) was found to be 82.33% and 80.07%, respectively. An accelerated study indicated that optimized formulation was stable.
Different transcriptional profiling between senescent and non-senescent human coronary artery endothelial cells (HCAECs) by Omeprazole and Lansoprazole treatment.[Pubmed:28039570]
Biogerontology. 2017 Apr;18(2):217-236.
Recent evidence suggests that high dose and/or long term use of proton pump inhibitors (PPIs) may increase the risk of adverse cardiovascular events in older patients, but mechanisms underlying these detrimental effects are not known. Taking into account that the senescent endothelial cells have been implicated in the genesis or promotion of age-related cardiovascular disease, we hypothesized an active role of PPIs in senescent cells. The aim of this study is to investigate the changes in gene expression occurring in senescent and non-senescent human coronary artery endothelial cells (HCAECs) following Omeprazole (OPZ) or Lansoprazole (LPZ) treatment. Here, we show that atherogenic response is among the most regulated processes in PPI-treated HCAECs. PPIs induced down-regulation of anti-atherogenic chemokines (CXCL11, CXCL12 and CX3CL1) in senescent but not in non-senescent cells, while the same chemokines were up-regulated in untreated senescent cells. These findings support the hypothesis that up-regulated anti-atherogenic chemokines may represent a defensive mechanism against atherosclerosis during cellular senescence, and suggest that PPIs could activate pro-atherogenic pathways by changing the secretory phenotype of senescent HCAECs. Moreover, the genes coding for fatty acid binding protein 4 (FABP4) and piezo-type mechanosensitive ion channel component 2 (PIEZO2) were modulated by PPIs treatment with respect to untreated cells. In conclusions, our results show that long-term and high dose use of PPI could change the secretory phenotype of senescent cells, suggesting one of the potential mechanisms by which use of PPI can increase adverse outcomes in older subjects.
Vonoprazan 20 mg vs lansoprazole 30 mg for endoscopic submucosal dissection-induced gastric ulcers.[Pubmed:27909552]
World J Gastrointest Endosc. 2016 Nov 16;8(19):716-722.
AIM: To compare the healing effects of vonoprazan and Lansoprazole on gastric ulcers induced by endoscopic submucosal dissection (ESD). METHODS: Data were obtained from a total of 26 patients. Fourteen patients were randomized to the vonoprazan group and 12 were randomized to the Lansoprazole group. Patients were administered either 20 mg vonoprazan or 30 mg Lansoprazole per day after ESD. Endoscopic images just after ESD, on day 8, and on day 28 were used for the evaluation of the shrinking rate of ESD ulcers. The shrinking rates and the incidence of delayed bleeding were compared between the 2 groups. RESULTS: The shrinking rates of ESD ulcers on day 8 [vonoprazan group: 61.8% (range: 24.0%-91.1%), Lansoprazole group: 71.3% (range: 25.2%-88.6%)] and on day 28 [vonoprazan group: 95.3% (range: 76.2%-100%), Lansoprazole group: 97.2% (range: 81.1%-99.8%)] were not statistically different between the 2 groups. On day 28, most of the ulcers in both groups healed to more than 90%, whereas 3 of 14 (21.4%) in the vonoprazan group and 1 of 12 (8.3%) in the Lansoprazole group had delayed ulcer healing, which was not statistically different (P = 0.356). The frequency of delayed bleeding was 0 in the both groups. Taken together, there were no significant differences between the two drug groups. CONCLUSION: Our study indicates that vonoprazan is potent for the management of ESD ulcers although Lansoprazole is also sufficient and cost-effective.
Ingestibility and Formulation Quality of Lansoprazole Orally Disintegrating Tablets.[Pubmed:28044122]
J Pharm (Cairo). 2016;2016:6131608.
Objectives. We evaluated the ingestibility and formulation quality of one branded (formulation A) and five generic (formulations B, C, D, E, and F) Lansoprazole orally disintegrating (OD) tablets. Methods. Ingestibility, including the oral disintegrating time, taste, mouth feeling, and palatability, was examined by sensory testing in healthy subjects. Formulation qualities, including salivary stability, gastric acid resistance, and intestinal dissolution behavior, were examined. Results and Discussion. The oral disintegration time of formulation F (52 s) was significantly longer than that of other formulations (32-37 s). More than 90% of subjects did not experience bitterness with formulations A, E, and F, whereas 50% of subjects felt rough and powdery sensations with formulations B, C, and D. More than 80% of subjects suggested that formulations A, E, and F had good palatability. Ingestibility was different between formulations. OD tablets consist of enteric granules containing Lansoprazole, which is unstable in gastric acid. Enteric granules of each formulation were stable in artificial saliva and gastric juice. No differences were observed in dissolution behaviors among the formulations, indicating that the formulation quality of the formulations was almost equivalent. Conclusions. This study provides useful information for selecting branded or generic Lansoprazole OD tablets for individualized treatments.
Lansoprazole: an update of its place in the management of acid-related disorders.[Pubmed:11693467]
Drugs. 2001;61(12):1801-33.
Lansoprazole is an inhibitor of gastric acid secretion and also exhibits antibacterial activity against Helicobacter pylori in vitro. Current therapy for peptic ulcer disease focuses on the eradication of H. pylori infection with maintenance therapy indicated in those patients who are not cured of H. pylori and those with ulcers resistant to healing. Lansoprazole 30 mg combined with amoxicillin 1g, clarithromycin 250 or 500mg, or metronidazole 400 mg twice daily was associated with eradication rates ranging from 71 to 94%, and ulcer healing rates were generally >80% in well designed studies. In addition, it was as effective as omeprazole- or rabeprazole-based regimens which included these antimicrobial agents. Maintenance therapy with Lansoprazole 30 mg/day was significantly more effective than either placebo or ranitidine in preventing ulcer relapse. Importantly, preliminary data suggest that Lansoprazole-based eradication therapy is effective in children and the elderly. In the short-term treatment of patients with gastro-oesophageal reflux disease (GORD), Lansoprazole 15, 30 or 60 mg/day was significantly more effective than placebo, ranitidine 300 mg/day or cisapride 40 mg/day and similar in efficacy to pantoprazole 40 mg/day in terms of healing of oesophagitis. Lansoprazole 30 mg/day, omeprazole 20 mg/day and pantoprazole 40 mg/day all provided similar symptom relief in these patients. In patients with healed oesophagitis. 12-month maintenance therapy with Lansoprazole 15 or 30 mg/day prevented recurrence and was similar to or more effective than omeprazole 10 or 20 mg/day. Available data in patients with NSAID-related disorders or acid-related dyspepsia suggest that Lansoprazole is effective in these patients in terms of the prevention of NSAID-related gastrointestinal complications, ulcer healing and symptom relief. Meta-analytic data and postmarketing surveillance in >30,000 patients indicate that Lansoprazole is well tolerated both as monotherapy and in combination with antimicrobial agents. After Lansoprazole monotherapy commonly reported adverse events included dose-dependent diarrhoea, nausea/vomiting, headache and abdominal pain. After short-term treatment in patients with peptic ulcer, GORD, dyspepsia and gastritis the incidence of adverse events associated with Lansoprazole was generally < or = 5%. Similar adverse events were seen in long-term trials, although the incidence was generally higher (< or = 10%). When Lansoprazole was administered in combination with amoxicillin, clarithromycin or metronidazole adverse events included diarrhoea, headache and taste disturbance. In conclusion, Lansoprazole-based triple therapy is an effective treatment option for the eradication of H. pylori infection in patients with peptic ulcer disease. Preliminary data suggest it may have an important role in the management of this infection in children and the elderly. In the short-term management of GORD, Lansoprazole monotherapy offers a more effective alternative to histamine H2-receptor antagonists and initial data indicate that it is an effective short-term treatment option in children and adolescents. In adults Lansoprazole maintenance therapy is also an established treatment option for the long-term management of this chronic disease. Lansoprazole has a role in the treatment and prevention of NSAID-related ulcers and the treatment of acid-related dyspepsia; however, further studies are needed to confirm its place in these indications. Lansoprazole has emerged as a useful and well tolerated treatment option in the management of acid-related disorders.
The gastric H,K-ATPase blocker lansoprazole is an inhibitor of chloride channels.[Pubmed:10711360]
Br J Pharmacol. 2000 Feb;129(3):598-604.
1. It was postulated that swelling dependent chloride channels are involved in the proton secretion of parietal cells. Since omeprazole, Lansoprazole and its acid activated sulphenamide form AG2000 are structurally related to phenol derivatives known to block swelling dependent chloride channels, we set out to test, whether these substances--which are known to block the H,K-ATPase--could also lead to an inhibition of swelling-dependent chloride channels. Swelling-dependent chloride channels--characterized in many different cell types--show highly conserved biophysical and pharmacological features, therefore we investigated the effect of omeprazole, Lansoprazole and its acid activated sulphenamide form AG2000 on swelling-dependent chloride channels elicited in fibroblasts, after the reduction of the extracellular osmolarity. 2. Omeprazole, Lansoprazole and its acid activated sulphenamide form AG2000 are able to block swelling-dependent chloride channels (IClswell). 3. Lansoprazole and its protonated metabolite AG2000 act on at least two different sites of the IClswell protein: on an extracellular site which seems to be in a functional proximity to the nucleotide binding site, and on an intracellular site which allows the formation of disulfide-bridges. 4. The inhibition of the proton pump and the simultaneous blocking of chloride channels by omeprazole, Lansoprazole and its acid activated sulphenamide form AG2000, as described here could be an effective mode to restrict proton secretion in parietal cells.
Antisecretory and antiulcer activities of a novel proton pump inhibitor AG-1749 in dogs and rats.[Pubmed:2537418]
J Pharmacol Exp Ther. 1989 Feb;248(2):806-15.
The antisecretory and antiulcer activities of 2[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl]methyl] sulfinyl]-1H-benzimidazole (AG-1749) were investigated in dogs and rats. AG-1749 inhibited both the (H+ + K+)-adenosine triphosphatase activity in canine gastric microsomes and dibutyryl cyclic AMP-stimulated acid formation in isolated canine parietal cells and suppressed the acid secretion stimulated by histamine, pentagastrin, bethanechol or a peptone meal in Heidenhain pouch dogs; the ID50 values were between 0.2 and 0.7 mg/kg p.o. AG-1749 inhibited both the histamine-stimulated and the basal acid secretion in pylorusligated rats and prevented water immersion stress or aspirin-induced gastric lesions and mepirizole or cysteamine-induced duodenal ulcers in rats; the ID50 values were between 0.3 to 3.6 mg/kg p.o. or i.d. Furthermore, AG-1749 prevented gastric lesions induced by absolute ethanol or acidified aspirin, and accelerated the healing of acetic acid-induced gastric or duodenal ulcers in rats. The inhibitory potency of AG-1749 in dogs was much the same as that of omeprazole and about half that of ranitidine. However, it was about 2 to 10 times more potent than omeprazole and 4 to 34 times more potent than ranitidine in rats. These results suggest that AG-1749 exerts prominent antiulcer activities mainly by suppressing acid secretion via an inhibition of a proton pump in gastric parietal cells and partly by protecting the gastrointestinal mucosa against various ulcerative stimuli.


