LanceolarinCAS# 15914-68-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 15914-68-8 | SDF | Download SDF |
| PubChem ID | 5492234 | Appearance | Powder |
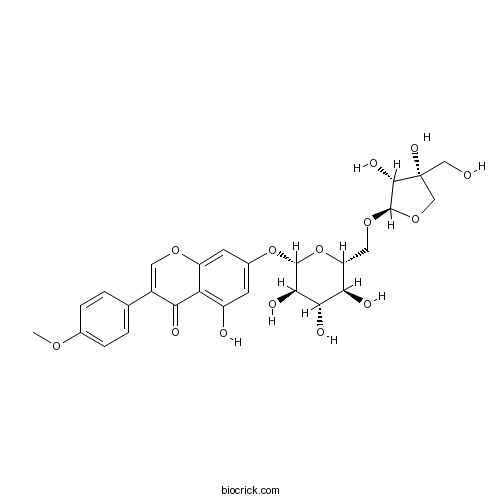
| Formula | C27H30O14 | M.Wt | 578.5 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 7-[(2S,3R,4S,5S,6R)-6-[[(2R,3R,4R)-3,4-dihydroxy-4-(hydroxymethyl)oxolan-2-yl]oxymethyl]-3,4,5-trihydroxyoxan-2-yl]oxy-5-hydroxy-3-(4-methoxyphenyl)chromen-4-one | ||
| SMILES | COC1=CC=C(C=C1)C2=COC3=CC(=CC(=C3C2=O)O)OC4C(C(C(C(O4)COC5C(C(CO5)(CO)O)O)O)O)O | ||
| Standard InChIKey | VGKGODYADVWBQB-NRIIMPDMSA-N | ||
| Standard InChI | InChI=1S/C27H30O14/c1-36-13-4-2-12(3-5-13)15-8-37-17-7-14(6-16(29)19(17)20(15)30)40-25-23(33)22(32)21(31)18(41-25)9-38-26-24(34)27(35,10-28)11-39-26/h2-8,18,21-26,28-29,31-35H,9-11H2,1H3/t18-,21-,22+,23-,24+,25-,26-,27-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | Zhongguo Zhong Yao Za Zhi. 2009 Aug;34(15):1921-6.Constituents of Millettia nitida var. hirsutissima.[Pubmed: 19894535]To separate effective constituents from Millettia nitida var. hirsutissima.
|

Lanceolarin Dilution Calculator

Lanceolarin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7286 mL | 8.643 mL | 17.2861 mL | 34.5722 mL | 43.2152 mL |
| 5 mM | 0.3457 mL | 1.7286 mL | 3.4572 mL | 6.9144 mL | 8.643 mL |
| 10 mM | 0.1729 mL | 0.8643 mL | 1.7286 mL | 3.4572 mL | 4.3215 mL |
| 50 mM | 0.0346 mL | 0.1729 mL | 0.3457 mL | 0.6914 mL | 0.8643 mL |
| 100 mM | 0.0173 mL | 0.0864 mL | 0.1729 mL | 0.3457 mL | 0.4322 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Specioside B
Catalog No.:BCN8784
CAS No.:126589-95-5
- 7-(4-hydroxy-3-methoxyphenyl)-1-phenylhept-4-en-3-one (DPHB)
Catalog No.:BCN8782
CAS No.:79559-60-7
- Dehydrojuncusol
Catalog No.:BCN8780
CAS No.:117824-04-1
- Isonardosinone
Catalog No.:BCN8779
CAS No.:27062-01-7
- Maculosidin
Catalog No.:BCN8778
CAS No.:522-19-0
- Silychristin B
Catalog No.:BCN8777
CAS No.:879325-58-3
- Yuanhunine
Catalog No.:BCN8776
CAS No.:104387-15-7
- 3-O-[5'''-O-feruloyl-beta-D-apiofuranosyl(1'''->2'')-beta-D-glucopyranosyl] rhamnocitrin
Catalog No.:BCN8775
CAS No.:148210-00-8
- Licoricesaponin H2(18beta,20alpha-Glycyrrhizic acid)
Catalog No.:BCN8774
CAS No.:118441-85-3
- Citrusin B
Catalog No.:BCN8773
CAS No.:105279-10-5
- Genistein 7-O-beta-D-glucopyranoside-4'-O-[alpha-L-rhamnopyranosyl-(1->2)-beta-D-glucopyranoside]
Catalog No.:BCN8772
CAS No.:70404-42-1
- 1,7-Diphenyl-5-hydroxy-4,6-hepten-3-one
Catalog No.:BCN8771
CAS No.:87095-77-0
- 5-Hydroxy-1-(4-hydroxyphenyl)-7-phenyl-3-heptanone (AO 2210)
Catalog No.:BCN8786
CAS No.:105955-04-2
- 1-Phenyl-2-propanol
Catalog No.:BCN8787
CAS No.:14898-87-4
- Puerol B
Catalog No.:BCN8788
CAS No.:112343-17-6
- Gardenin D
Catalog No.:BCN8789
CAS No.:29202-00-4
- Emodin-8-O-beta-gentiobioside
Catalog No.:BCN8790
CAS No.:66466-22-6
- 5-Hydroxy-7-(4'-hydroxy-3'-methoxyphenyl)-1-phenyl-3-heptanone (DHPA)
Catalog No.:BCN8791
CAS No.:79559-61-8
- 4-Hydroxybenzoyl choline
Catalog No.:BCN8793
CAS No.:5094-31-5
- Patulitrin
Catalog No.:BCN8794
CAS No.:19833-25-1
- 7-(4-Hydroxyphenyl)-1-phenyl-4-hepten-3-one
Catalog No.:BCN8795
CAS No.:100667-52-5
- 3,7,25-Trihydroxycucurbita-5,23-dien-19-al
Catalog No.:BCN8799
CAS No.:85372-65-2
- Yuankanin
Catalog No.:BCN8755
CAS No.:77099-20-8
- New biochemical 1
Catalog No.:BCN8781
CAS No.:
[Absorption and transport of isoflavonoid compounds from Tongmai formula across human intestinal epithelial (Caco-2) cells in vitro].[Pubmed:29171242]
Zhongguo Zhong Yao Za Zhi. 2017 Aug;42(16):3206-3212.
Tongmai formula (TMF) is a drug combination of three components including Puerariae Lobatae Radix [roots of Pueraria lobata], Salviae Miltiorrhizae Radix (roots of Salvia miltiorrhiza) and Chuanxiong Rhizoma (rhizomes of Ligusticum chuanxiong) in a weight ratio of 1ratio1ratio1. The absorption and transport of isoflavonoid compounds from Tongmai formula across human intestinal epithelial (Caco-2) cells in vitro were studied in this paper. The assay isoflavonoid compounds include daidzein, formononetin, 5-hydroxylononin, ononin, daidzin, 3'-methoxypuerarin, genistin, puerarin, formononetin-8-C-beta-D-apiofuranosyl-(1-->6)-O-beta-D-glucopyranoside, formononetin-7-O-beta-D-apiofuranosyl-(1-->6)-O-beta-D-glucopyranoside, Lanceolarin, kakkanin, daidzein-7,4'-di-O-beta-D-glucopyranoside, mirificin, 3'-hydroxypuerarin, 3'-methoxydaidzin, formononetin-8-C-beta-D-xylopyranosyl-(1-->6)-O-beta-D-glucopyranoside, genistein-8-C-beta-D-apiofuranosyl-(1-->6)-O-beta-D-glucopyranoside, genistein-7-O-beta-D-apiofuranosyl-(1-->6)-O-beta-D-glucopyranoside (ambocin), 3'-hydroxymirificin, 6''-O-beta-D-xylosylpuerarin, biochanin A-8-C-beta-D-apiofuranosyl-(1-->6)-O-beta-D-glucopyranoside, 3'-methoxydaidzein-7,4'-di-O-beta-D-glucopyranoside, daidzein-7-O-beta-D-glucopyranosyl-(1-->4)-O-beta-D-glucopyranoside, and daidzein-7-O-alpha-D-glucopyranosyl-(1-->4)-O-beta-D-glucopyranoside. By using human Caco-2 monolayer as an intestinal epithelial cell model in vitro, the permeability of above-mentioned 25 isoflavonoids in TMF were studied from the apical (AP) side to basolateral (BL) side or from the BL side to AP side. The assay compounds were determined by reversed phased high-performance liquid chromatography (HPLC) coupled with UV detector. Transport parameters and apparent permeability coefficients (Papp) were then calculated and and compared with those of propranolol and atenolol, which are the transcellular transport marker and as a control substance for high and poor permeability, respectively. The Papp values of daidzein and formononetin were (2.55+/-0.03) x10(-)(5),(3.06+/-0.01) x10(-)(5) cm*s(-)(1) from AP side to BL side, respectively, and (2.62+/-0.00) x10(-)(5), (2.65+/-0.11) x10(-)(5) cm*s(-)(1) from BL side to AP side, respectively. Under the condition of this experiment, the Papp value was (2.66+/-0.32) x10(-)(5) cm*s(-)(1) for propranolol and (2.34+/-0.10) x10(-)(7) cm*s(-)(1) for atenolol. The Papp values of daidzein and formononetin were at a same magnitude with those of propranolol. And the Papp values of other 23 isoflavonoid compounds were at a same magnitude with those of atenolol. On the other hand, the rats of Papp AP-->BL/Papp BL-->AP of daidzein and formononetin on the influx transport were 0.97 and 1.15, respectively. It can be predicted that daidzein and formononetin can be absorbed across intestinal epithelial cells to go to the body circulation by the passive diffusion mechanism and they were assigned to the well-absorbed compounds. Other 23 isoflavonoid compounds were assigned to the poorly absorbed compounds. Because of the rats of Papp AP-->BL/Papp BL-->AP of 5-hydroxylononin, genistin, Lanceolarin, kakkanin, and genistein-7-O-beta-D-apiofuranosyl-(1-->6)-O-beta-D-glucopyranoside were 0.18, 0.28, 0.45, 0.38, 0.49, they may have been involved in the efflux mechanism in Caco-2 cells monolayer model from the BL side to AP side direction.
[Constituents of Millettia nitida var. hirsutissima].[Pubmed:19894535]
Zhongguo Zhong Yao Za Zhi. 2009 Aug;34(15):1921-6.
OBJECTIVE: To separate effective constituents from Millettia nitida var. hirsutissima. METHOD: Compounds were isolated by chromatography methods, structures were identified by spectroscopic means. RESULT: Eight flavonoids (1-8) and two triterpenes (9-10) were isolated from this plant. They were identified as calycosin (1), genistin (2), gliricidin (3), 8-O-methylretusin (4), afromosin-7-O-beta-D-glucopyranoside (5), Lanceolarin (6), soliquiritigenin (7), symplocoside (8), lupeol (9), 3beta-friedelanol (10). CONCLUSION: The compounds (1-10) were obtained from M. nitida var. hirsutissima for the first time. The 13C-NMR dada of 1 were correct assignment on the basis of 2D-NMR spectral analysis.
[Isoflavones from Millettia nitida var. hirsutissima].[Pubmed:19408686]
Yao Xue Xue Bao. 2009 Feb;44(2):158-61.
To study the chemical constituents of Millettia nitida var. hirsutissima, the constituents were isolated by chromatographic techniques, and structures were identified by spectroscopic methods. Eight isoflavones were isolated and identified, including a new compound, hirsutissimiside F (1), and seven known compounds, formononetin (2), ononin (3), odoratin 7-O-beta-D-glucopyranoside (4), Lanceolarin (5), afromosin (6), sphaerobioside (7), and hirsutissimiside B (8). Compounds 3, 4, 5 and 7 were isolated from the genus Millettia for the first time, 2 was obtained from this plant for the first time.
Biochanin A triglycoside from Andira inermis.[Pubmed:11077173]
Fitoterapia. 2000 Dec;71(6):663-7.
A new isoflavonol triglycoside, biochanin A 7-O-beta-D-apiofuranosyl-(1-->5)-beta-D-apiofuranosyl-(1-->6 )-beta-D- glucopyranoside (1), was isolated from Andira inermis roots in addition to the known compounds genistein 7-O-beta-D-apiofuranosyl-(1-->6)-beta-D-glucopyranoside and Lanceolarin.


